
CHAPTER 4
Chemical Foundations: Elements,
Atoms, and Ions
1.
fire, earth, water, air
2.
Robert Boyle
3.
Boyle’s most important contribution was his insistence that science should be firmly grounded in
experiment. Boyle tried to limit the influence of any preconceptions about science and only
accepted as fact what could be demonstrated.
4.
oxygen, carbon, hydrogen
5.
From Table 4.1: oxygen, silicon, aluminum, iron, calcium
6.
a.
Trace elements are those elements which are present in only tiny amounts in the body,
but are critical for many bodily processes and functions.
b.
Answer depends on your choice of elements
7.
B (boron); C (carbon); F (fluorine); H (hydrogen); I (iodine); K (potassium); N (nitrogen); O
(oxygen); P (phosphorus); S (sulfur); U (uranium); V (vanadium); W (tungsten); Y (yttrium)
8.
Sometimes the symbol for an element is based on its common name in another language. This is
true for many of the more common metals since their existence was known to the ancients: some
examples are iron, sodium, potassium, silver, and tin (the symbols come from their name in
Latin); tungsten (the symbol comes from its name in German).
9.
a.
4
b.
11
c.
12
d.
3
e.
8
f.
9
g.
13
h.
7
i.
6
j.
5
10.
a.
neon
b.
nickel
c.
nitrogen
d.
nobelium
43

Chapter 4: Elements, Atoms, and Ions
e.
neptunium
f.
niobium
g.
neodymium
11.
Co
cobalt
Rb
rubidium
Rn
radon
Ra
radium
U
uranium
12.
Zr
zirconium
Cs
cesium
Se
selenium
Au
gold
Ce
cerium
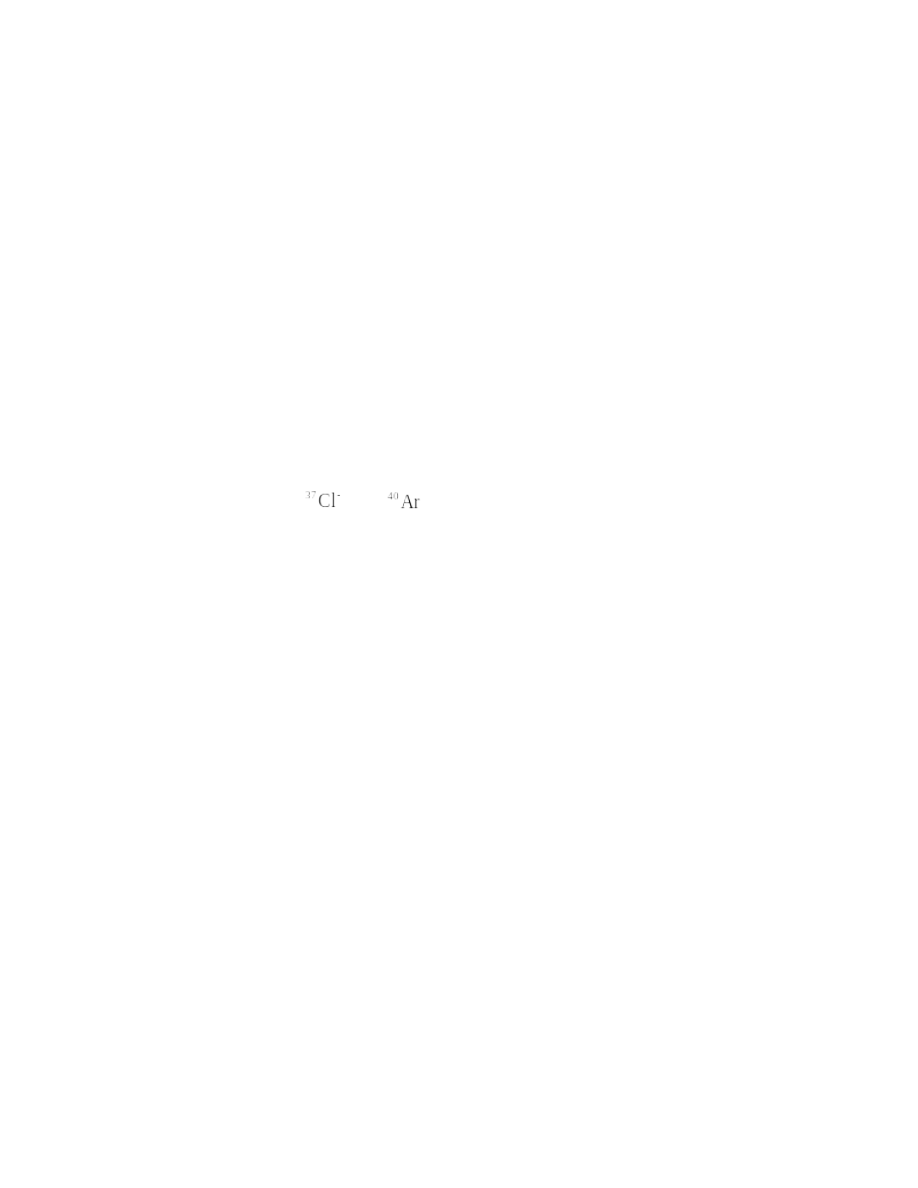
13.
a.
potassium
b.
germanium
c.
phosphorus
d.
carbon
e.
nitrogen
f.
sodium
g.
neon
h.
iodine
14.
B: barium, Ba; berkelium, Bk; beryllium, Be; bismuth, Bi; bohrium, Bh; boron, B; bromine, Br
N: neodymium, Nd; neon, Ne; neptunium, Np; nickel, Ni; niobium, Nb; nitrogen, N;
nobelium, No
P: palladium, Pd; phosphorus, P; platinum, Pt; plutonium, Pu; polonium, Po; potassium, K;
praseodymium, Pr; promethium, Pm; protactinium, Pa
S: samarium, Sm; scandium, Sc; seaborgium, Sg; selenium, Se; silicon, Si; silver, Ag; sodium,
Na; strontium, Sr; sulfur, S
15.
the law of constant composition
16.
a.
Elements are made of tiny particles called atoms.
b.
All the atoms of a given element are identical
c.
The atoms of a given element are different from those of any other element.
d.
A given compound always has the same numbers and types of atoms.
e.
Atoms are neither created nor destroyed in chemical processes. A chemical
reaction simply changes the way the atoms are grouped together.
44

Chapter 4: Elements, Atoms, and Ions
17.
A compound is a distinct substance that is composed of two or more elements and always
contains exactly the same relative masses of those elements.
18.
According to Dalton, all atoms of the same element are identical; in particular, every atom of a
given element has the same mass as every other atom of that element. If a given compound
always contains the same relative numbers of atoms of each kind, and those atoms always have
the same masses, then it follows that the compound made from those elements would always
contain the same relative masses of its elements.
19.
a.
C6H6
d.N2O4
b.
AlCl3
e.
NaHCO3
c.
Na2S
f.
KI
20.
a.
CO2
d.
H2SO4
b.
CO
e.
BaCl2
c.
CaCO3
f.
Al2S3
21.
a.
J. J. Thomson discovered the electron. Thomson postulated that, because negative
particles had been detected in the atom, then there must also be positive particles to
counterbalance the negative charge.
b.
William Thomson (Lord Kelvin) described the atom as a uniform pudding of
positive charge, with electrons scattered throughout (like the raisins in a pudding)
to balance the electrical charge.
22.
False. Rutherford’s bombardment experiments with metal foil suggested that the alpha particles
were being deflected by coming near a dense, positively charged atomic nucleus.
23.
Neutrons are found in the nucleus and carry no electrical charge.
24.
protons
25.
The proton and the neutron have similar (but not identical) masses. Both of these particles have a
mass approximately 2000 times greater than that of an electron. The combination of the protons
and the neutrons make up the bulk of the mass of an atom, but the electrons make the greatest
contribution to the chemical properties of the atom.
26.
neutron; electron
27.
10–13 cm = 10–15 m
28.
the electrons; outside the nucleus; Because they are located in the exterior regions of the atom, it
is the electrons of an atom that most interact with other atoms and are therefore most responsible
for the atom’s chemical behavior.
29.
Although all atoms of a given element contain the same number of protons in the nucleus, some
atoms of a given element may have different numbers of neutrons. Isotopes are atoms of the same
element with different mass numbers.
30.
False. The mass number represents the total number of protons and neutrons in the nucleus.
31.
An isolated atom has no charge, therefore the number of negatively charged electrons must equal
the number of positively charged protons.
32.
Neutrons are uncharged and contribute only to the mass.
45

Chapter 4: Elements, Atoms, and Ions
33.
Dalton’s original assumption was reasonable for his time, but as mass determination techniques
improved, it was discovered that a given element may be composed of several isotopes. Isotopes
have the same number of protons and electrons, and so are chemical identical, but differ in the
number of neutrons, which causes some physical differences.
34.
James Chadwick
35.
Z
Symbol
Name
8
O
oxygen
29
Cu
copper
78
Pt
platinum
15
P
phosphorus
17
Cl
chlorine
50
Sn
tin
30
Zn
zinc
36.
(a) and (b); Isotopes are atoms with the same number of protons but different numbers of
neutrons. In a nuclide symbol, the bottom number represents the number of protons in the atom.
The top number represents the mass number, which is the sum of the number of neutrons and
number of protons in the atom. Thus, both a and b have the same number of protons (10 protons)
but different numbers of neutrons (a contains 10 neutrons and b contains 12 neutrons).
37.
a.
13
6 C
b.
12
6 C
c.
14
6 C
d.
11
5 B
e.
10
5 B
f.
10
5 B
38.
a.
54
26 Fe
b.
56
26 Fe
c.
57
26 Fe
d.
14
7 N
e.
15
7 N
f.
15
7 N
39.
a.
56 protons, 74 neutrons, 56 electrons
b.
56 protons, 80 neutrons, 56 electrons
c.
22 protons, 24 neutrons, 22 electrons
d.
22 protons, 26 neutrons, 22 electrons
e.
3 protons, 3 neutrons, 3 electrons
f.
3 protons, 4 neutrons, 3 electrons
46

Chapter 4: Elements, Atoms, and Ions
40.
The relative amounts of 2H and 18O in a person’s hair, compared to other isotopes of these
elements, vary significantly from region to region in the United States and is related to the
isotopic abundances in the drinking water in a region.
41.
Isotopes are atoms that contain the same number of protons but a different number of neutrons.
The text gives an example in which ivory from African elephants was identified as coming from a
specific region based on the ratio of 13C to 12C in the ivory.
42.
Name
Symbol
Atomic Number
Mass Number
Number of neutrons
oxygen
17
8 O
8
17
9
oxygen
17
8 O
8
17
9
neon
20
10 Ne
10
20
10
iron
56
26 Fe
26
56
30
plutonium
244
94 Pu
94
244
150
mercury
202
80 Hg
80
202
122
cobalt
59
27 Co
27
59
32
nickel
56
28 Ni
28
56
28
fluorine
19
9 F
9
19
10
chromium
50
24 Cr
24
50
26
43.
False. The elements are listed in the periodic table in order of increasing atomic number (number
of protons in the nucleus; nuclear charge), so that elements with similar properties form vertical
groups.
44.
Elements with similar chemical properties are aligned vertically in families known as groups.
45.
Metals are excellent conductors of heat and electricity, and are malleable, ductile, and generally
shiny (lustrous) when a fresh surface is exposed.
46.
Metallic elements are found toward the left and bottom of the periodic table. There are far more
metallic elements than there are nonmetals.
47.
Mercury is a liquid at room temperature.
48.
The gaseous nonmetallic elements are hydrogen, nitrogen, oxygen, fluorine, chlorine, plus all the
group 8 elements (noble gases). There are no gaseous metallic elements under room conditions.
49.
The only metal that ordinarily occurs as a liquid is mercury. The only nonmetallic element that
occurs as a liquid at room temperature is bromine (elements such as oxygen and nitrogen are
frequently obtainable as liquids, but these result from compression of the gases into cylinders at
very low temperatures).
50.
metalloids or semimetals
51.
a.
Group 1; alkali metals
b.
Group 2; alkaline earth elements
c.
Group 8; noble gases
d.
Group 7; halogens
e.
Group 2; alkaline earth elements
47

Chapter 4: Elements, Atoms, and Ions
f.
Group 8; noble gases
g.
Group 1; alkali metals
52.
a.
fluorine, chlorine, bromine, iodine, astatine
b.
lithium, sodium, potassium, rubidium, cesium, francium
c.
beryllium, magnesium, calcium, strontium, barium, radium
d.
helium, neon, argon, krypton, xenon, radon
53.
a.
Sr; Z = 38; Group 2; metal
b.
I; Z = 53; Group 7; nonmetal
c.
Si; Z = 14; Group 4; metalloid
d.
Cs; Z = 55; Group 1; metal
e.
S; Z = 16; Group 6; nonmetal
54.
Arsenic, atomic number 33, is located on the dividing line between the metallic elements and the
non-metallic elements, and is therefore classified as a metalloid. Arsenic is in Group 5 of the
periodic table, whose other principal members are N, P, Sb, and Bi.
55.
compounds (and mixtures of compounds)
56.
Most of the elements are too reactive to be found in the uncombined form in nature and are found
only in compounds.
57.
argon
58.
These elements are found uncombined in nature and do not readily react with other elements. For
many years it was thought that these elements formed no compounds at all, although this has now
been shown to be untrue.
59.
diatomic
60.
diatomic gases: H2, N2, O2, Cl2, and F2
monatomic gases: He, Ne, Kr, Xe, Rn, and Ar
61.
electricity
62.
chlorine
63.
liquids: bromine, mercury, gallium
gases: hydrogen, nitrogen, oxygen, fluorine, chlorine, and the noble gases (helium, neon, argon,
krypton, xenon, radon)
64.
graphite
65.
zero
66.
electrons
67.
loses three
68.
2–
69.
cations, anions
70.
-ide
48

Chapter 4: Elements, Atoms, and Ions
71.
The answer will depend on the student’s selection of elements; in general, the metallic elements
are the ones that form positively charged ions.
72.
False. N3– contains 7 protons and 10 electrons. P3– contains 15 protons and 18 electrons.
73.
a.
54
d.
10
b.
18
e.
54
c.
23
f.
80
74.
number of protons = 8; number of electrons = 10; number of neutrons = 9
75.
a.
Ca: 20 protons, 20 electrons
Ca2+: 20 protons, 18 electrons
b.
P: 15 protons, 15 electrons
P3–: 15 protons, 18 electrons
c.
Br: 35 protons, 35 electrons
Br–: 35 protons, 36 electrons
d.
Fe: 26 protons, 26 electrons
Fe3+: 26 protons, 23 electrons
e.
Al: 13 protons, 13 electrons
Al3+: 13 protons, 10 electrons
f.
N: 7 protons, 7 electrons
N3–: 7 protons, 10 electrons
76.
a.
two electrons gained
b.
three electrons gained
c.
three electrons lost
d.
two electrons lost
e.
one electron lost
f.
two electrons lost.
77.
a.
I–
b.
Sr2+
c.
Cs+
d.
Ra2+
e.
F–
f.
Al3+
78.
a.
P3–
b.
Ra2+
c.
At–
d.
no ion
e.
Cs+
f.
Se2–
79.
A compound that has a high melting point (many hundreds of degrees) and which conducts an
electrical current when melted or dissolved in water almost certainly consists of ions. Nonionic
compounds have lower melting points than ionic compounds and do not conduct electricity when
melted or in solution.
49

Chapter 4: Elements, Atoms, and Ions
80.
Sodium chloride is an ionic compound, consisting of Na+ and Cl– ions. When NaCl is dissolved in
water, these ions are set free and can move independently to conduct the electric current.
81.
In the solid state, although ions are present, they are rigidly held in fixed positions in the crystal
of the substance. In order for ionic substances to be able to pass an electrical current, the ions
must be able to move, which is possible when the solid is converted to the liquid state.
82.
The total number of positive charges must equal the total number of negative charges so that
there will be no net charge on the crystals of an ionic compound. A macroscopic sample of
compound must ordinarily not have any net charge.
83.
a.
KCl, K2S, K3N
b.
MgCl2, MgS, Mg3N2
c.
AlCl3, Al2S3, AlN
d.
CaCl2, CaS, Ca3N2
e.
LiCl, Li2S, Li3N
84.
a.
CsI, BaI2, AlI3
b.
Cs2O, BaO, Al2O3
c.
Cs3P, Ba3P2, AlP
d.
Cs2Se, BaSe, Al2Se3
e.
CsH, BaH2, AlH3
85.
a.
At; Z = 85
e. Pb; Z = 82
b.
Xe; Z = 54
f.
Se; Z = 34
c.
Ra; Z = 88
g.
Ar; Z = 18
d.
Sr; Z = 38
h.
Cs; Z = 55
86.
a.
7; halogens
b.
8; noble gases
c.
2; alkaline earth elements
d.
2; alkaline earth elements
e.
4
f.
6; (the members of group 6 are sometimes called the chalcogens)
g.
8; noble gases
h.
1; alkali metals
50
Chapter 4: Elements, Atoms, and Ions
87.
Element
Symbol
Atomic Number
Group 1
hydrogen
H
1
lithium
Li
3
sodium
Na
11
potassium
K
19
Group 2
beryllium
Be
4
magnesium
Mg
12
calcium
Ca
20
strontium
Sr
38
Group 6
oxygen
O
8
sulfur
S
16
selenium
Se
34
tellurium
Te
52
Group 7
fluorine
F
9
chlorine
Cl
17
bromine
Br
35
iodine
I
53
88.
(b); Dalton’s atomic theory stated that all atoms of a given element are identical.
89.
(d);
Group Number:
7A
8A
# of Protons:
17
18
# of Neutrons:
20
22
# of Electrons:
18
18
90.
Most of the mass of an atom is concentrated in the nucleus: the protons and neutrons that
constitute the nucleus have similar masses, and these particles are nearly two thousand times
heavier than electrons. The chemical properties of an atom depend on the number and location of
the electrons it possesses. Electrons are found in the outer regions of the atom and are the
particles most likely to be involved in interactions between atoms.
91.
Yes. For example, carbon and oxygen form carbon monoxide (CO) and carbon dioxide (CO2).
The existence of more than one compound between the same elements does not in any way
contradict Dalton’s theory. For example, the relative mass of carbon in different samples of CO is
always the same, and the relative mass of carbon in different samples of CO2 is also always the
same. Dalton did not say, however, that two different compounds would have to have the same
relative masses of the elements present. In fact, Dalton said that two different compounds of the
same elements would have to have different relative masses of the elements.
92.
C6H12O6
93.
FeO and Fe2O3
94.
a.
29 protons; 34 neutrons; 29 electrons
b.
35 protons; 45 neutrons; 35 electrons
c.
12 protons; 12 neutrons; 12 electrons
51

Chapter 4: Elements, Atoms, and Ions
95.
Mass Number Symbol
Number of Neutrons
24
24
13 Al 11
25
25
13 Al 12
26
26
13 Al 13
28
28
13 Al 15
29
29
13 Al 16
30
30
13 Al 17
They are all considered aluminum atoms because the identity of the element is defined by the
atomic number, which is the same for all of the isotopes listed.
96.
The chief use of gold in ancient times was as ornamentation, whether in statuary or in jewelry.
Gold possesses an especially beautiful luster, and because it is relatively soft and malleable, it
could be worked finely by artisans. Among the metals, gold is particularly inert to attack by most
substances in the environment.
97.
Boyle defined a substance as an element if it could not be broken down into simpler substances
by chemical means.
98.
a.
36
b.
36
c.
21
d.
36
e.
80
f.
27
99.
a.
Ba
b.
K
c.
Cs
d.
Pb
e.
Pt
f.
Au
100.
(e); B, Si, and Ge are considered metalloids or semimetals.
101.
a.
Ag
b.
Al
c.
Cd
d.
Sb
e.
Sn
f.
As
52

Chapter 4: Elements, Atoms, and Ions
102.
The metal ion is Cu2+. Since the metal ion has 27 electrons and contains a 2+ charge, this means
that it has two less electrons as compared to protons. Therefore the number of protons is 29. The
number of protons is also the atomic number, identifying the metal ion as copper. Mass number =
29 p+ + 34 n = 63
103.
a.
tellurium
b.
palladium
c.
zinc
d.
silicon
e.
cesium
f.
bismuth
g.
fluorine
h.
titanium
104.
a.
CO2
b.
AlCl3
c.
HClO4
d.
SCl6
105.
a.
nitrogen, N
b.
neon, Ne
c.
sodium, Na
d.
nickel, Ni
e.
titanium, Ti
f.
argon, Ar
g.
krypton, Kr
h.
xenon, Xe
106.
a.
13
6 C
b.
13
6 C
c.
13
6 C
d.
44
19 K
e.
41
20 Ca
f.
35
19 K
107.
a.
22 protons, 19 neutrons, 22 electrons
b.
30 protons, 34 neutrons, 30 electrons
c.
32 protons, 44 neutrons, 32 electrons
d.
36 protons, 50 neutrons, 36 electrons
53

Chapter 4: Elements, Atoms, and Ions
e.
33 protons, 42 neutrons, 33 electrons
f.
19 protons, 22 neutrons, 19 electrons
108.
Symbol
Protons
Neutrons
Mass Number
41
20 Ca
20
21
41
55
25 Mn
25
30
55
109
47 Ag
47
62
109
45
21Sc
21
24
45
109.
a.
C; Z = 6; nonmetal
b.
Se; Z = 34; nonmetal
c.
Rn; Z = 86; nonmetal; noble gases
d.
Be; Z = 4; metal; alkaline earth elements
110.
Cu-63: 29 protons, 29 electrons, 34 neutrons,
Cu-65: 29 protons, 29 electrons, 36 neutrons,
111.
Au: gold
Kr: krypton
He: helium
C: carbon
Li: lithium
Si: silicon
112.
tin: Sn
beryllium: Be
hydrogen: H
chlorine: Cl
radium: Ra
xenon: Xe
zinc: Zn
oxygen: O
113.
# Protons
# Neutrons
Symbol
34
45
Se
19
20
K
53
74
I
4
5
Be
24
32
Cr
114.
Atom
G or L
Ion
O
G
O2–
Mg
L
Mg2+
Rb
L
Rb+
Br
G
Br–
Cl
G
Cl–
54

Chapter 4: Elements, Atoms, and Ions
115.
Atoms
# Protons
# Neutrons
25
30
8
10
28
31
92
146
80
121
116.
Atom/Ion
Protons
Neutrons
Electrons
50
70
50
12
13
10
26
30
24
34
45
34
17
18
17
29
34
29
117.
(a); Rutherford is the founder of the nuclear atom. A proton is heavier than an electron.
The nucleus contains protons and neutrons.
55