
CHAPTER 15
Solutions
1.
Specific answers depend on student choices.
2.
A heterogeneous mixture does not have a uniform composition: the composition varies in
different places within the mixture. Examples of non–homogeneous mixtures include salad
dressing (mixture of oil, vinegar, water, herbs, and spices) and granite (combination of minerals).
3.
Water is the solvent, sugar the solute.
4.
solvent; solutes
5.
When an ionic solute dissolves in water, a given ion is pulled into solution by the attractive ion–
dipole force exerted by several water molecules. For example, in dissolving a positive ion, the ion
is approached by the negatively charged end of several water molecules: if the attraction of the
water molecules for the positive ion is stronger than the attraction of the negative ions near it in
the crystal, the ion leaves the crystal and enters solution. After entering solution, the dissolved ion
is surrounded completely by water molecules, which tends to prevent the ion from reentering the
crystal.
6.
“Like dissolves like.” The hydrocarbons in oil have intermolecular forces that are very different
from those in water, and so the oil spreads out rather than dissolving in the water.
7.
Salts are composed of positive and negative ions packed closely together in a crystal lattice. The
crystal is held together by the ionic forces among oppositely charged particles in the solid. When
a crystal of a salt is placed in water, the polar water molecules arrange their dipoles around the
ions of the crystal in such a way as to attract the ions. For example, a positive ion at a corner of
the crystal will be surrounded by a group of water molecules aiming the negative end of their
dipoles at the positive ion. If the forces of attraction of the water molecules for the positive ion
are larger than the ionic forces holding the ion in the crystal, then the ion will leave the crystal
and enter solution. See Figure 15.2
8.
Carbon dioxide is somewhat soluble in water, especially if pressurized (otherwise, the soda you
may be drinking while studying Chemistry would be “flat”). Carbon dioxide’s solubility in water
is approximately 1.5 g/L at 25°C under a pressure of approximately 1 atm. Although the carbon
dioxide molecule overall is non-polar, that is because the two individual C–O bond dipoles cancel
each other due to the linearity of the molecule. However these bond dipoles are able to interact
with water, making CO2 more soluble in water than non-polar molecules such as O2 or N2 that do
not possess individual bond dipoles.
9.
A saturated solution is one that contains the maximum amount of solute possible at a particular
temperature. A saturated solution is one that is in equilibrium with undissolved solute.
10.
unsaturated
11.
variable
316

Chapter 15: Solutions
12.
large
13.
The mass percent of a particular component in a solution is the mass of that component, divided
by the total mass of the solution, multiplied by 100%.
14.
100.0
15.
a.
2.14 g KCl
100 =
(2.14 g KCl + 12.5 g water)
14.6% KCl
b.
2.14 g KCl
100 =
(2.14 g KCl + 25.0 g water)
7.89% KCl
c.
2.14 g KCl
100 =
(2.14 g KCl + 37.5 g water)
5.40% KCl
d.
2.14 g KCl
100 =
(2.14 g KCl + 50.0 g water)
4.10% KCl
16.
a.
b.
c.
d.
2.00 mg = 0.00200 g
17.
a.
375 g ×
4
1.51 g NH Cl
100. g solution
= 5.66 g NH4Cl
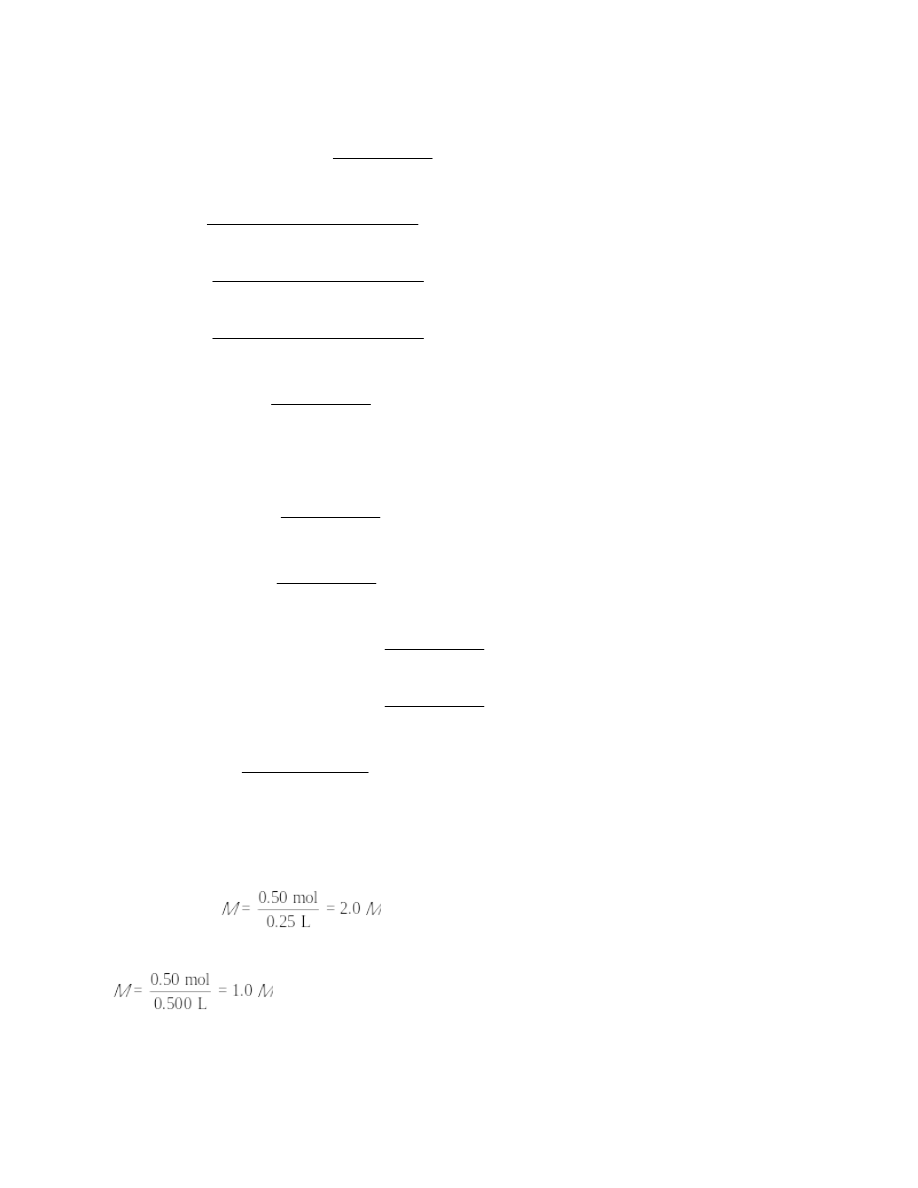
b.
125 g ×
2.91 g NaCl
100. mg solution
= 3.64 g NaCl
c.
1.31 kg = 1.31 × 103 g
1.31 × 103 g ×
3
4.92 g KNO
100. g solution
= 64.5 g KNO3
d.
478 mg = 0.478 g
0.478 g ×
4
3
12.5 g NH NO
100. g solution
= 0.0598 g NH4NO3
317
Chapter 15: Solutions
18.
a.
525 g solution ×
3
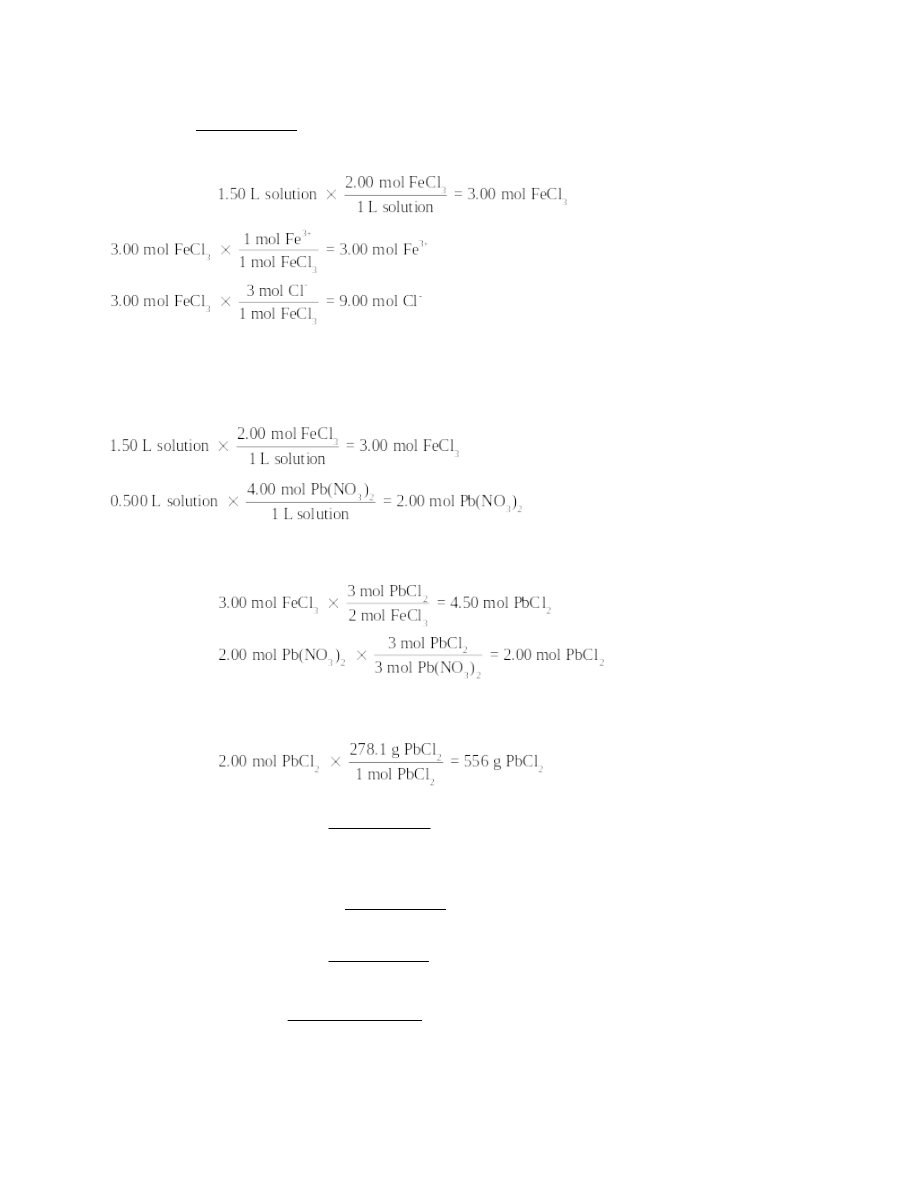
3.91 g FeCl
100.g solution
= 20.5 g FeCl3
525 g solution – 20.5 g FeCl3 = 504.5 g water (505 g water)
b.
225 g solution ×
11.9 g sucrose
100. g solution
= 26.8 g sucrose
225 g solution – 26.8 g sucrose = 198.2 g water (198 g water)
c.
1.45 kg = 1.45 × 103 g
1.45 × 103 g solution ×
12.5 g NaCl
100. g solution
= 181.3 g NaCl (181 g NaCl)
1.45 × 103 g solution – 181.3 g NaCl = 1268.7 g water (1.27 × 103 g water)
d.
635 g solution ×
3
15.1 g KNO
100. g solution
= 95.9 g KNO3
635 g solution – 95.9 g KNO3 = 539.1 g water (539 g water)
19.
Total mass = 92.1 g + 2.59 g + 1.59 g = 96.28 g
%Fe =
92.1 g Fe
100
96.28 g alloy
95.7% Fe
%C =
2.59 g C
100
96.28 g alloy
2.69% C
%Cr =
1.59 g Cr
100
96.28 g alloy
1.65% Cr
20.
Percent means “per hundred”. So the percentages in Question 19 represent the number of grams
of the particular element present in 100. g of the alloy. Since 1.00 kg represents ten times the
mass of 100. g, we would need 957 g Fe, 26.9 g C, and 16.5 g Cr to prepare 1.00 kg of the alloy.
21.
To say that the solution contains 7.51% of ammonium nitrate means that every 100. grams of the
solution will contain 7.51 g of ammonium nitrate.
1.25 kg = 1.25 × 103 g
1.25 × 103 g solution
4
3
7.51 g NH NO
100 g solution
= 93.9 g NH4NO3
1.25 × 103 g solution – 93.9 g NH4NO3 = 1156.1 g water (1.16 × 103 g water)
22.
2
2
67.1 g CaCl
(67.1 g CaCl + 275 g water)
100 = 19.6% CaCl2
318
Chapter 15: Solutions
23.
To say that the solution to be prepared is 4.50% by mass CaCl2 means that 4.50 g of CaCl2 will be
contained in every 100. g of the solution.
175 g solution
2
4.50 g CaCl
100. g solution
= 7.88 g CaCl2
24.
To say that the solution is 6.25% KBr by mass, means that 100. g of the solution will contain 6.25
g KBr.
125 g solution
6.25 g KBr
100. g solution
= 7.81 g KBr
25.
285 g solution
5.00 g NaCl
100.0 g solution
= 14.3 g NaCl
285 g solution
2
3
7.50 g Na CO
100.0 g solution
= 21.4 g Na2CO3
26.
molar mass O2 = 32.00 g
1.00 g O2 ×
1 mol
32.00 g
= 0.03125 mol O
from the balanced chemical equation, it will take 2(0.03125) = 0.0625 mol H2O2 to produce this
quantity of oxygen.
molar mass H2O2 = 34.02 g
0.0625 mol H2O2 ×
2
2
2
2
34.02 g H O
1 mol H O
= 2.13 g H2O2
2.13 g H2O2 ×
2
2
100. g solution
3 g H O
= approximately 71 g
27.
First find the mass of the solution, because the percentage is by mass.
1.00 L = 1.00 × 103 mL
1.00 × 103 mL solution
1.84 g solution
1 mL solution
= 1840 g solution = 1.84 × 103 g solution
1.84 × 103 g solution
2
4
98.3 g H SO
100. g solution
= 1.81 × 103 g H2SO4
28.
1000 g ×
0.95 g stablizer
100. g
= 9.5 g
29.
Each liter of the solution contains 3 mol of hydrogen chloride.
30.
0.110 mol; 0.220 mol
319
Chapter 15: Solutions
31.
A standard solution is one whose concentration is known very accurately and with high precision.
A standard solution is typically prepared by weighing out a precise amount of solute, and then
dissolving the solute in a precise amount of solvent, or to a precise final volume using a
volumetric flask.
32.
The molarity represents the number of moles of solute per liter of solution: choice b is the only
scenario that fulfills this definition.
33.
Molarity =
moles of solute
liters of solution
a.
125 mL = 0.125 L
0.521 mol
0.125 L
M
4.17 M
b.
250. mL = 0.250 L
M =
0.521 mol
0.250 L
= 2.08 M
c.
500. mL = 0.500 L
M =
0.521 mol
0.500 L
= 1.04 M
d.
M =
0.521 mol
1.00 L
= 0.521 M
34.
Molarity =
moles of solute
liters of solution
a.
225 mL = 0.225 L
M =
3
0.754 mol KNO
0.225 L
= 3.35 M
b.
10.2 mL = 0.0102 L
M =
2
0.0105 mol CaCl
0.0102 L
= 1.03 M
c.
M =
3.15 mol NaCl
5.00 L
= 0.630 M
d.
100. mL = 0.100 L
M =
0.499 mol NaBr
0.100 L
= 4.99 M
35.
Molarity =
moles of solute
liters of solution
; molar mass of NaCl = 58.44 g
3.51 g NaCl ×
1 mol NaCl
58.44 g NaCl
= 0.06006 mol NaCl
320
Chapter 15: Solutions
a.
25 mL = 0.025 L
M =
0.06006 mol NaCl
0.025 L
= 2.4 M
b.
50. mL = 0.050 L
M =
0.06006 mol
0.050 L
= 1.2 M
c.
75 mL = 0.075 L
M =
0.06006 mol
0.075 L
= 0.80 M
d.
M =
0.06006 mol
1.00 L
= 0.0601 M
36.
Molarity =
moles of solute
liters of solution
; molar mass of CaCl2 = 110.98 g; 125 mL = 0.125 L
a.
5.59 g CaCl2
2
2
1 mol CaCl
110.98 g CaCl
= 0.05037 mol CaCl2
M =
0.05037 mol
0.125 L
= 0.403 M
b.
2.34 g CaCl2
2
2
1 mol CaCl
110.98 g CaCl
= 0.02108 mol CaCl2
M =
0.02108 mol
0.125 L
= 0.169 M
c.
8.73 g CaCl2
2
2
1 mol CaCl
110.98 g CaCl
= 0.07866 mol CaCl2
M =
0.07866 mol
0.125 L
= 0.629 M
d.
11.5 g CaCl2
2
2
1 mol CaCl
110.98 g CaCl
= 0.1036 mol CaCl2
M =
0.1036 mol
0.125 L
= 0.829 M
37.
molar mass of CaCl2 = 110.98 g; 225 mL = 0.225 L
0.225 L ×
0.150 mol 110.98 g
1 L
1 mol
3.75 g CaCl2
38.
molar mass of NH4Cl = 53.492 g; 450. mL = 0.450 L
0.450 L
0.251 mol
1 L
53.492 g
1 mol
= 6.04 g NH
4Cl
321
Chapter 15: Solutions
39.
molar mass CaCO3 = 100.1 g; 250.0 mL = 0.2500 L
1.745 g CaCO3
1 mol
100.1 g
= 0.01743 mol CaCO3
One mole of CaCO3 contains one mole of Ca2+ ion; therefore,
M =
2+
0.01743 mol Ca
0.2500 L solution
= 0.06973 M
40.
molar mass of I2 = 253.8 g; 225 mL = 0.225 L
5.15 g I2
1 mol
253.8 g
= 0.0203 mol I2
M =
2
0.0203 mol I
0.225 L solution
= 0.0902 M
41.
molar mass of NaOH = 40.00 g; 225 mL = 0.225 L
42.5 g NaOH
1 mol NaOH
40.00 g
= 1.063 mol NaOH
M =
1.063 mol NaOH
0.225 L solution
= 4.72 M
42.
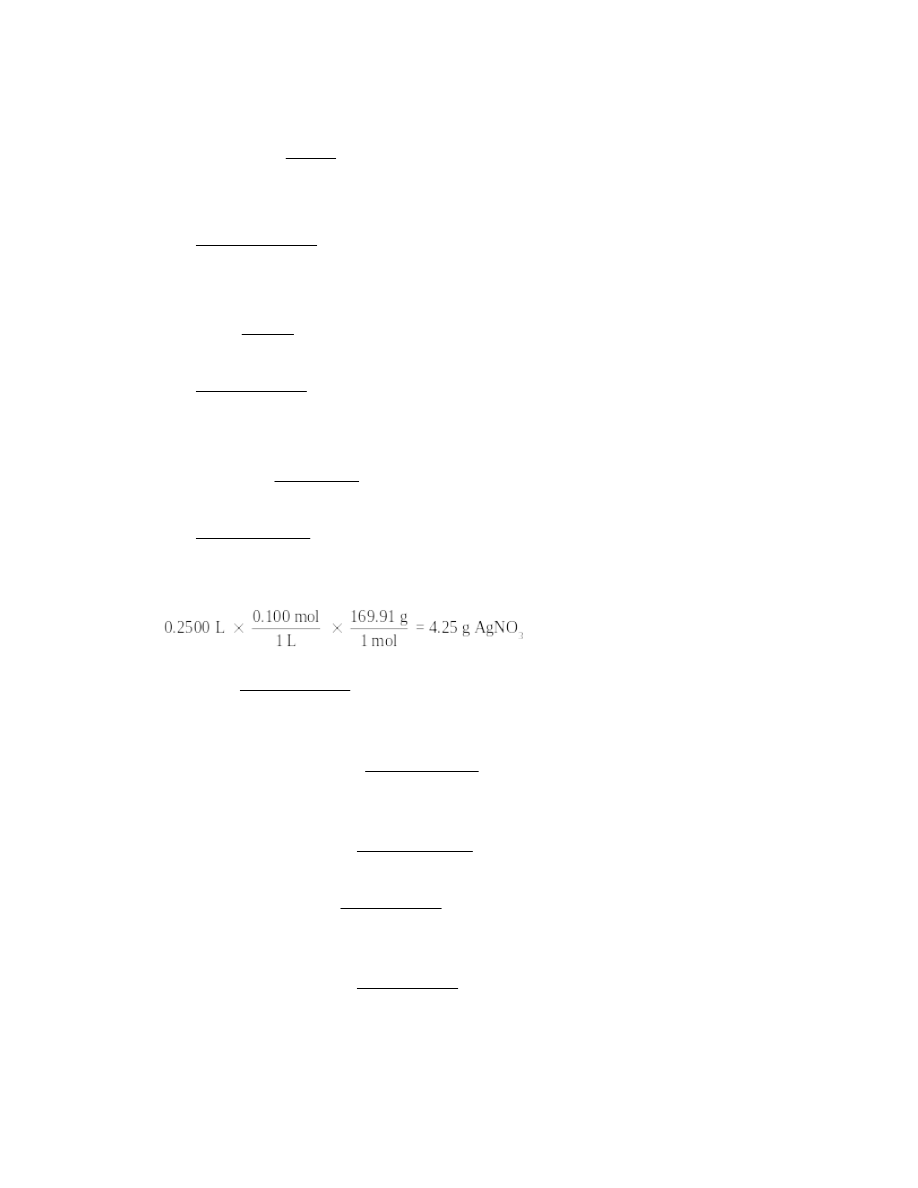
molar mass of AgNO3 = 169.91 g; 250. mL = 0.2500 L
43.
Molarity =
moles of solute
liters of solution
a.
4.25 mL = 0.00425 L
0.00425 L solution
2
0.105 mol CaCl
1.00 L solution
= 4.46 10–4 mol CaCl2
b.
11.3 mL = 0.0113 L
0.0113 L solution
0.405 mol NaOH
1.00 L solution
= 4.58 10–3 mol NaOH
c.
1.25 L solution
12.1 mol HCl
1.00 L solution
= 15.1 mol HCl
d.
27.5 mL = 0.0275 L
0.0275 L solution
1.98 mol NaCl
1.00 L solution
= 0.0545 mol NaCl
322
Chapter 15: Solutions
44.
a.
12.5 mL = 0.0125 L
0.0125 L solution
0.104 mol HCl
1.00 L solution
= 0.00130 mol HCl
b.
27.3 mL = 0.0273 L
0.0273 L solution
0.223 mol NaOH
1.00 L solution
= 0.00609 mol NaOH
c.
36.8 mL = 0.0368 L
0.0368 L solution
3
0.501 mol HNO
1.00 L solution
= 0.0184 mol HNO3
d.
47.5 mL = 0.0475 L
0.0475 L solution
0.749 mol KOH
1.00 L solution
= 0.0356 mol KOH
45.
a.
2.50 L solution
13.1 mol HCl
1.00 L solution
= 32.75 mol HCl
molar mass HCl = 36.46 g
32.75 mol HCl
36.46 g HCl
1 mol HCl
= 1194 g HCl = 1.19 103 g HCl
b.
15.6 mL = 0.0156 L
0.0156 L solution
0.155 mol NaOH
1.00 L solution
= 0.002418 mol NaOH
molar mass NaOH = 40.00 g
0.002418 mol NaOH
40.00 g NaOH
1 mol
= 0.0967 g NaOH
c.
135 mL = 0.135 L
0.135 L solution
3
2.01 mol HNO
1.00 L solution
= 0.2714 mol HNO3
molar mass HNO3 = 63.02 g
0.2714 mol HNO3
3
63.02 g HNO
1 mol
= 17.1 g HNO3
d.
4.21 L solution
2
0.515 mol CaCl
1.00 L solution
= 2.168 mol CaCl2
molar mass CaCl2 = 111.0 g
2.168 mol CaCl2
2
111.0 g CaCl
1 mol
= 241 g CaCl2
323
Chapter 15: Solutions
46.
a.
molar mass of CaCl2 = 110.98 g; 17.8 mL = 0.0178 L
0.0178 L solution
2
2
2
0.119 mol CaCl
110.98 g CaCl
1 L solution
1 mol CaCl
= 0.235 g CaCl2
b.
molar mass of KCl = 74.55 g; 27.6 mL = 0.0276 L
0.0276 L solution
0.288 mol KCl
74.55 g KCl
1 L solution
1 mol KCl
= 0.593 g KCl
c.
molar mass of FeCl3 = 162.2 g; 35.4 mL = 0.0354 L
0.0354 L solution
3
3
3
0.399 mol FeCl
162.2 g FeCl
1 L solution
1 mol FeCl
= 2.29 g FeCl3
d.
molar mass KNO3 = 101.11 g; 46.1 mL = 0.0461 L
0.0461 L solution
3
3
3
0.559 mol KNO
101.11 g KNO
1 L solution
1 mol KNO
= 2.61 g KNO3
47.
molar mass of NaOH = 40.00 g
3.5 L solution
0.50 mol NaOH
40.00 g NaOH
1 L solution
1 mol NaOH
= 70. g NaOH
48.
molar mass of KBr = 119.0 g; 225 mL = 0.225 L
0.225 L solution
0.355 mol KBr
119.0 g KBr
1 L solution
1 mol KBr
= 9.51 g KBr
49.
a.
1.00 L solution
2
4
0.251 mol Na SO
1.00 L
= 0.251 mol Na2SO4
0.251 mol Na2SO4
+
2
4
2 mol Na
1 mol Na SO
= 0.502 mol Na+
b.
5.50 L solution
3
0.1 mol FeCl
1.00 L
= 0.550 mol FeCl3
0.550 mol FeCl3
3
3 mol Cl
1 mol FeCl
= 1.65 mol = 1.7 mol Cl–
c.
100. mL = 0.100 L
0.100 L solution
3 2
0.55 mol Ba(NO )
1.00 L
= 0.0550 mol Ba(NO3)2
0.0550 mol Ba(NO3)2
3
3 2
2 mol NO
1 mol Ba(NO )
= 0.11 mol NO3–
324
Chapter 15: Solutions
d.
250. mL = 0.250 L
0.250 L solution
4 2
4
0.350 mol (NH ) SO
1.00 L
= 0.0875 mol (NH4)2SO4
0.0875 mol (NH4)2SO4
4
4 2
4
2 mol NH
1 mol (NH ) SO
= 0.175 mol NH4+
50.
a.
10.2 mL = 0.0102 L
0.0102 L
3+
3
3
0.451 mol AlCl
1 mol Al
1.00 L
1 mol AlCl
= 4.60 10–3 mol Al3+
0.0102 L
3
3
0.451 mol AlCl
3 mol Cl
1.00 L
1 mol AlCl
= 1.38 10–2 mol Cl–
b.
5.51 L
+
3
4
3
4
0.103 mol Na PO
3 mol Na
1.00 L
1 mol Na PO
= 1.70 mol Na+
5.51 L
3
3
4
4
3
4
0.103 mol Na PO
1 mol PO
1.00 L
1 mol Na PO
= 0.568 mol PO43–
c.
1.75 mL = 0.00175 L
0.00175 L
2
2
2
1.25 mol CuCl
1 mol Cu
1.00 L
1 mol CuCl
= 2.19 10–3 mol Cu2+
0.00175 L
2
2
1.25 mol CuCl
2 mol Cl
1.00 L
1 mol CuCl
= 4.38 10–3 mol Cl–
d.
25.2 mL = 0.0252 L
0.0252 L
2+
2
2
0.00157 mol Ca(OH)
1 mol Ca
1.00 L
1 mol Ca(OH)
= 3.96 10–5 mol Ca2+
0.0252 L
2
2
0.00157 mol Ca(OH)
2 mol OH
1.00 L
1 mol Ca(OH)
= 7.91 10–5 mol OH–
51.
molar mass NaCl = 58.44 g; 125 mL = 0.125 L
0.125 L ×
0.105 mol NaCl
58.44 g NaCl
1.00 L
1 mol
= 0.767 g NaCl for 125 mL of the solution
0.105 mol NaCl
58.44 g NaCl
1.00 L
1 mol
= 6.14 g NaCl for 1.00 L of the solution
52.
Molar mass of Na2CO3 = 106.0 g; 250 mL = 0.250 L
0.250 L
2
3
0.0500 mol Na CO
106.g
1.00 L
1 mol
= 1.33 g Na2CO3
53.
moles of solute
325

Chapter 15: Solutions
54.
half
55.
1
1
2
2
M
V
M
V
a.
M1 = 0.119 M
M2 = ?
V1 = 25.0 mL = 0.0250 L
V2 = (55.0 + 25.0) mL = 80.0 mL = 0.0800 L
M2 =
(0.119 )(0.0250 L)
(0.0800 L)
M
= 0.0372 M
b.
M1 = 0.701 M
M2 = ?
V1 = 45.3 mL = 0.0453 L
V2 = (45.3 + 125) = 170.3 mL = 0.1703 L
M2 =
(0.701 )(0.0453 L)
(0.1703 L)
M
= 0.186 M
c.
M1 = 3.01 M
M2 = ?
V1 = 125 mL = 0.125 L
V2 = (125 + 550.) mL = 675 mL = 0.675 L
M2 =
(3.01 )(0.125 L)
(0.675 L)
M
= 0.557 M
d.
M1 = 2.07 M
M2 = ?
V1 = 75.3 mL = 0.0753 L
V2 = (75.3 + 335) mL = 410.3 mL = 0.4103 L
M2 =
(2.07 )(0.0753 L)
(0.4103 L)
M
= 0.380 M
56.
1
1
2
2
M
V
M
V
a.
M1 = 0.251 M
M2 = ?
V1 = 125 mL
V2 = (125 + 250.) = 375 mL
b.
M1 = 0.499 M
M2 = ?
V1 = 445 mL
V2 = (445 + 250.) = 695 mL
c.
M1 = 0.101 M
M2 = ?
V1 = 5.25 L
V2 = (5.25 + 0.250) = 5.50 L
d.
M1 = 14.5 M
M2 = ?
V1 = 11.2 mL
V2 = (11.2 + 250.) = 261.2 mL
326

Chapter 15: Solutions
57.
1
1
2
2
M
V
M
V
HCl:
(3.0 )(225 mL)
(12.1 )
M
M
= 55.8 mL = 56 mL
HNO3:
(3.0 )(225 mL)
(15.9 )
M
M
= 42.45 mL = 42 mL
H2SO4:
(3.0 )(225 mL)
(18.0 )
M
M
= 37.5 mL = 38 mL
HC2H3O2:
(3.0 )(225 mL)
(17.5 )
M
M
= 38.6 mL = 39 mL
H3PO4:
(3.0 )(225 mL)
(14.9 )
M
M
= 45.3 mL = 45 mL
58.
M1 = 19.4 M
M2 = 3.00 M
V1 = ? mL
V2 = 3.50 L
M1 =
(3.00 )(3.50 mL)
(19.4 )
M
M
= 0.541 L (541 mL)
59.
1
1
2
2
M
V
M
V
M1 = 2.00 M
M2 = 0.350 M
V1 = ?
V2 = 275 mL = 0.275 L
V1 =
(0.350 )(0.275 L)
(2.00 )
M
M
= 0.0481 L = 48.1 mL
Dilute 48.1 mL of the 2.00 M solution to a final volume of 275 mL.
60.
1
1
2
2
M
V
M
V
M1 = 1.01 M
M2 = 0.150 M
V1 = ? mL
V2 = 325 mL
M2 =
(0.150 )(325 mL)
(1.01 )
M
M
= 48.3 mL
Dilute 48.3 mL of the 1.01 M solution to a final volume of 325 mL.
61.
1
1
2
2
M
V
M
V
M1 = 0.200 M
M2 = 0.150 M
V1 = 500. mL = 0.500 L
V2 = ?
327
Chapter 15: Solutions
V2 =
(0.200 )(0.500 L)
(0.150 )
M
M
= 0.667 L = 667 mL
Therefore 667 – 500. = 167 mL of water must be added.
62.
(100. mL)(1.25 )
(12.1
)
M
M
= 10.3 mL
63.
10.0 mL = 0.0100 L
The number of moles of Ni2+ ion present is given by
0.0100 L ×
2+
0.103 mol Ni
1 L
= 0.00103 mol Ni2+
Since Ni2+ and DMG react on a 1:2 mole basis, then 2 × 0.00103 = 0.00206 mol of DMG are
needed for the reaction
0.00206 mol DMG ×
1 L
0.0703 mol DMG
= 0.0293 L = 29.3 mL of the DMG solution is needed.
64.
Na2CO3(aq) + CaCl2(aq) CaCO3(s) + 2NaCl(s)
mmol Ca2+ ion: 37.2 mL ×
2+
0.105 mmol Ca
1.00 mL
= 3.91 mmol Ca2+
From the balanced chemical equation, 3.91 mmol CO32 will be needed to precipitate this quantity
of Ca2+ ion.
3.91 mmol CO32 ×
1.00 mL
0.125 mmol
= 31.2 mL
65.
The balanced net ionic equation for the precipitation reaction is
Cu2+(aq) + S2–(aq) CuS(s)
copper ion present: 27.5 mL
2+
0.121 mmol Cu
1.00 mL
= 3.328 mmol Cu2+
As the coefficients of the balanced equation are both 1, then 3.328 mmol of S2– ion will be
required to precipitate the Cu2+ ion.
volume sulfide solution: 3.328 mmol S2–
2
1.00 mL
0.105 mmol S
= 31.7 mL Na2S
66.
molar mass Na2C2O4 = 134.0 g
37.5 mL = 0.0375 L
moles Ca2+ ion = 0.0375 L
2+
0.104 mol Ca
1.00 L
= 0.00390 mol Ca2+ ion
Ca2+(aq) + C2O42–(aq) CaC2O4(s)
328
Chapter 15: Solutions
As the precipitation reaction is of 1:1 stoichiometry, then 0.00390 mol of C2O42– ion is needed.
Moreover, each formula unit of Na2C2O4 contains one C2O42– ion, so 0.00390 mol of Na2C2O4 is
required.
0.00390 mol Na2C2O4
134.0 g
1 mol
= 0.523 g Na2C2O4 required
67.
Pb(NO3)2(aq) + K2CrO4(aq) PbCrO4(s) + 2KNO3(aq)
molar masses: Pb(NO3)2, 331.2 g; PbCrO4, 323.2 g
1.00 g Pb(NO3)2
3 2
3 2
1 mol Pb(NO )
331.2 g Pb(NO )
= 0.003019 mol Pb(NO3)2
25.0 mL = 0.0250 L
0.0250 L solution
2
4
1.00 mol K CrO
1.00 L solution
= 0.0250 mol K2CrO4
Pb(NO3)2 is the limiting reactant: 0.003019 mol PbCrO4 will form.
0.003019 mol PbCrO4
4
4
323.2 g PbCrO
1 mol PbCrO
= 0.976 g PbCrO4
68.
10.0 mL = 0.0100 L
0.0100 L
3
0.250 mol AlCl
1.00 L
= 2.50 10–3 mol AlCl3
AlCl3(aq) + 3NaOH(s) Al(OH)3(s) + 3NaCl(aq)
2.50 10–3 mol AlCl3
3
3 mol NaOH
1 mol AlCl
= 7.50 10–3 mol NaOH
molar mass NaOH = 40.00 g
7.50 10–3 mol NaOH
40.00 g NaOH
1 mol
= 0.300 g NaOH
69.
NaOH(aq) + HNO3(aq) NaNO3(aq) + H2O(l)
27.2 mL = 0.0272 L
0.0272 L
3
0.491 mol HNO
1 L
= 0.01335 mol HNO3
0.01335 mol HNO3
3
1 mol NaOH
1 mol HNO
= 0.01335 mol NaOH
0.01335 mol NaOH
1 L solution
0.502 mol NaOH
= 0.0226 L = 26.6 mL
70.
NaOH(aq) + HCl(aq) H2O(l) + NaCl(aq)
125 mL = 0.125 L
329

Chapter 15: Solutions
0.125 L
3.01 mol NaOH
1 L
= 0.3763 mol NaOH
0.3763 mol NaOH
1 mol HCl
1 mol NaOH
= 0.3763 mol HCl
0.3763 mol HCl
1 L solution
0.995 mol HCl
= 0.378 L = 378 mL
71.
HCl(aq) + NaHCO3(s) NaCl(aq) + H2O(l) + CO2(g)
molar mass of NaHCO3 = 84.01 g; 47.21 mL = 0.04721 L
0.1015 g NaHCO3 ×
1 mol
84.01 g
= 0.001208 mol NaHCO3
The balanced chemical equation indicates that HCl and NaHCO3 react on a 1:1 basis, so 0.001208
mol of HCl must be present. Therefore, the molarity of the HCl is given by
M =
0.001208 mol
0.04721 L
= 0.02559 M
72.
7.2 mL = 0.0072 L
0.0072 L
-3
2.5 10 mol NaOH
1.00 L
= 1.8 10–5 mol NaOH
H+(aq) + OH–(aq) H2O(l)
1.8 10–5 mol OH–
+
1 mol H
1 mol OH
= 1.8 10–5 mol H+
100 mL = 0.100 L
M =
5
+
1.8 10 mol H
0.100 L
= 1.8 10–4 M H+(aq)
73.
a.
NaOH(aq) + HC2H3O2(aq) NaC2H3O2(aq) + H2O(l)
25.0 mL = 0.0250 L
0.0250 L
0.154 mol acetic acid
1.00 L
= 0.00385 mol acetic acid
0.00385 mol acetic acid
1 mol NaOH
1 mol acetic acid
= 0.00385 mol NaOH
0.00385 mol NaOH
1.00 L
1.00 mol NaOH
= 0.00385 L = 3.85 mL NaOH
b.
HF(aq) + NaOH(aq) NaF(aq) + H2O(l)
35.0 mL = 0.0350 L
0.0350 L
0.102 mol HF
1.00 L
= 0.00357 mol HF
330
Chapter 15: Solutions
0.00357 mol HF
1 mol HF
1 mol NaOH
= 0.00357 mol NaOH
0.00357 mol NaOH
1.00 L
1.00 mol NaOH
= 0.00357 L = 3.57 mL
c.
H3PO4(aq) + 3NaOH(aq) Na3PO4(aq) + 3H2O(l)
10.0 mL = 0.0100 L
0.0100 L
3
4
0.143 mol H PO
1.00 L
= 0.00143 mol H3PO4
0.00143 mol H3PO4
3
4
3 mol NaOH
1 mol H PO
= 0.00429 mol NaOH
0.00429 mol NaOH
1.00 L
1.00 mol NaOH
= 0.00429 L = 4.29 mL
d.
H2SO4(aq) + 2NaOH(aq) Na2SO4(aq) + 2H2O(l)
35.0 mL = 0.0350 L
0.0350 L
2
4
0.220 mol H SO
1.00 L
= 0.00770 mol H2SO4
0.00770 mol H2SO4
2
4
2 mol NaOH
1 mol H SO
= 0.0154 mol NaOH
0.0154 mol NaOH
1.00 L
1.00 mol NaOH
= 0.0154 L = 15.4 mL
74.
Experimentally, neutralization reactions are usually performed with volumetric glassware that is
calibrated in milliliters rather than liters. For convenience in calculations for such reactions, the
arithmetic is often performed in terms of milliliters and millimoles, rather than in liters and moles:
1 mmol = 0.001 mol. Note that the number of moles of solute per liter of solution, the molarity, is
numerically equivalent to the number of millimoles of solute per milliliter of solution.
a.
HNO3(aq) + NaOH(aq) NaNO3(aq) + H2O(l)
12.7 mL
0.501 mmol
1.00 mL
= 6.36 mmol NaOH present in the sample
6.36 mmol NaOH
3
1 mmol HNO
1 mmol NaOH
= 6.36 mmol HNO3 required to react
6.36 mmol HNO3
3
1.00 mL
0.101 mmol HNO
= 63.0 mL HNO3 required
b.
2HNO3(aq) + Ba(OH)2 Ba(NO3)2 + 2H2O(l)
24.9 mL
0.00491 mmol
1.00 mL
= 0.122 mmol Ba(OH)2 present in sample
0.122 mmol Ba(OH)2
3
2
2 mmol HNO
1 mmol Ba(OH)
= 0.244 mmol HNO3 required
331

Chapter 15: Solutions
0.244 mmol HNO3
3
1.00 mL
0.101 mmol HNO
= 2.42 mL HNO3 is required
c.
HNO3(aq) + NH3(aq) NH4NO3(aq)
49.1 mL
0.103 mmol
1.00 mL
= 5.06 mmol NH3 present in the sample
5.06 mmol NH3
3
3
1 mmol HNO
1 mmol NH
= 5.06 mmol HNO3 required
5.06 mmol HNO3
3
1.00 mL
0.101 mmol HNO
= 50.1 mL HNO3 required
d.
KOH(aq) + HNO3(aq) KNO3(aq) + H2O(l)
1.21 L
0.102 mol
1.00 L
= 0.123 mol KOH present in the sample
0.123 mol KOH
3
1 mol HNO
1 mol KOH
= 0.123 mol HNO3 required
0.123 mol HNO3
3
1.00 L
0.101 mol HNO
= 1.22 L HNO3 required
75.
one mole of protons (hydrogen ions)
76.
1 normal
77.
When H2SO4 reacts with OH–, the reaction is
H2SO4(aq) + 2OH–(aq) 2H2O(l) + SO42–(aq).
As each mol of H2SO4 provides two moles of H+ ion, it is only necessary to take half a mole of
H2SO4 to provide one mole of H+ ion. The equivalent weight of H2SO4 is thus half the molar
mass.
78.
1.53 equivalents OH– ion are needed to react with 1.53 equivalents of H+ ion. By definition, one
equivalent of OH– ion exactly neutralizes one equivalent of H+ ion.
79.
N =
number of equivalents of solute
number of liters of solution
a.
equivalent weight HCl = molar mass HCl = 36.46 g
M2 =
1 1
2
(25.2 mL)(0.105 )
(75.3 mL)
M V
M
V
= 0.0351 M = 0.0351 N
b.
equivalent weight H3PO4 =
molar mass
3
3
4
3
4
3 equivalents H PO
0.253 mol H3PO4
1.00 L
1 mole H PO
= 0.759 N
332

Chapter 15: Solutions
c.
equivalent weight Ca(OH)2 =
molar mass
2
2
2
2
0.00103 mol Ca(OH)
2 equivalents Ca(OH)
1.00 L
1 mol Ca(OH)
= 0.00206 N
80.
N =
number of equivalents of solute
number of liters of solution
a.
equivalent weight NaOH = molar mass NaOH = 40.00 g
0.113 g NaOH
1 equiv NaOH
40.00 g
= 2.83 10–3 equiv NaOH
10.2 mL = 0.0102 L
N =
3
2.83 10 equiv
0.0102 L
= 0.277 N
b.
equivalent weight Ca(OH)2
molar mass
74.10 g
2
2
= 37.05 g
12.5 mg
3
1 g
1 equiv
10 mg 37.05 g
= 3.37 10–4 equiv Ca(OH)2
100. mL = 0.100 L
N =
4
3.37 10 equiv
0.100 L
= 3.37 10–3 N
c.
equivalent weight H2SO4 =
molar mass
98.09 g
=
2
2
= 49.05 g
12.4 g
1 equiv
49.05 g
= 0.253 equiv H2SO4
155 mL = 0.155 L
N =
0.253 equiv
0.155 L
= 1.63 N
81.
a.
0.250 M HCl
1 equiv HCl
1 mol HCl
= 0.250 N HCl
b.
0.105 M H2SO4
2
4
2
4
2 equiv H SO
1 mol H SO
= 0.210 N
c.
5.3 10–2 M H3PO4
3
4
3
4
3 equiv H PO
1 mol H PO
= 0.159 N = 0.16 N
333

Chapter 15: Solutions
82.
a.
0.134 M NaOH
1 equiv NaOH
1 mol NaOH
= 0.134 N NaOH
b.
0.00521 M Ca(OH)2
2
2
2 equiv Ca(OH)
1 mol Ca(OH)
= 0.0104 N Ca(OH)2
c.
4.42 M H3PO4
3
4
3
4
3 equiv H PO
1 mol H PO
= 13.3 N H3PO4
83.
molar mass H3PO4 = 98.0 g
35.2 g H3PO4
3
4
3
4
1 mol H PO
98.0 g H PO
= 0.3592 mol H3PO4
M =
3
4
0.3592 mol H PO
1.00 L
= 0.3592 M = 0.359 M
0.359 M H3PO4
3
4
3
4
3 equiv H PO
1 mol H PO
= 1.08 N H3PO4
84.
Molar mass of Ca(OH)2 = 74.10 g
5.21 mg Ca(OH)2
3
1 g
1 mol
10 mg 74.10 g
= 7.03 10–5 mol Ca(OH)2
1000. mL = 1.000 L (volumetric flask volume: 4 significant figures).
M =
5
7.03 10 mol
1.000 L
= 7.03 10–5 M Ca(OH)2
N = 7.03 10–5 M Ca(OH)2
2
2
2 equiv Ca(OH)
1 mol Ca(OH)
= 1.41 10–4 N Ca(OH)2
85.
H2SO4(aq) + 2NaOH(aq) Na2SO4(aq) + 2H2O(l)
Nacid Vacid = Nbase Vbase
(0.35 N) (15.0 mL) = (0.50 N) (Vbase)
Vbase = 10.5 mL = 11 mL
86.
H2SO4(aq) + 2NaOH(aq) Na2SO4(aq) + 2H2O(l)
Nacid Vacid = Nbase Vbase
(0.104 N)(Vacid) = (0.152 N)(15.2 mL)
Vacid = 22.2 mL
The 0.104 M sulfuric acid solution is twice as concentrated as the 0.104 N sulfuric acid solution
(1 mole = 2 equivalents), so half as much will be required to neutralize the same quantity of
NaOH = 11.1 mL
334
Chapter 15: Solutions
87.
2NaOH(aq) + H2SO4(aq) Na2SO4(aq) + 2H2O(l)
For the 0.125 N H2SO4:
Nacid Vacid = Nbase Vbase
(0.125 N) (24.2 mL) = (0.151 N) (Vbase)
Vbase = 20.0 mL of the 0.151 N NaOH solution needed
For the 0.125 M H2SO4:
As each H2SO4 formula unit produces two H+ ions, the normality of this solution will be twice its
molarity.
0.125 M H2SO4 = 0.250 N H2SO4
Nacid Vacid = Nbase Vbase
(0.250 N) (24.1 mL) = (0.151 N) (Vbase)
Vbase = 39.9 mL of the 0.151 N NaOH solution needed
88.
2NaOH(aq) + H2SO4(aq) Na2SO4(aq) + 2 H2O(l)
27.34 mL NaOH
0.1021 mmol
1.00 mL
= 2.791 mmol NaOH
2.791 mmol NaOH
2
1 mmol H SO4
2 mmol NaOH
= 1.396 mmol H2SO4
M =
2
1.396 mmol H SO4
25.00 mL
= 0.05583 M H2SO4 = 0.1117 N H2SO4
89.
total mass of solution = 50.0 g + 50.0 g + 5.0 g = 105.0 g
% ethanol =
50.0 g ethanol
105.0 g total
100 = 47.6% ethanol
% water =
50.0 g water
105.0 g total
100 = 47.6% water
% sugar =
5.0 g sugar
105.0 g total
100 = 4.8% sugar
1.5 g sugar
100.0 g solution
4.8 g sugar
= 31 g solution
10.0 g ethanol
100.0 g solution
47.6 g ethanol
= 21.0 g solution
90.
Molarity is defined as the number of moles of solute contained in 1 liter of total solution volume
(solute plus solvent after mixing). In the first case, where 50. g of NaCl is dissolved in 1.0 L of
water, the total volume after mixing is not known and the molarity cannot be calculated. In the
second example, the final volume after mixing is known and the molarity can be calculated
simply.
335
Chapter 15: Solutions
91.
mmol CoCl2 = 50.0 mL 0.250 M CoCl2 = 12.5 mmol CoCl2
This contains 12.5 mmol Co2+ and 25.0 mmol Cl–
mmol NiCl2 = 25.0 mL 0.350 M NiCl2 = 8.75 mmol NiCl2
This contains 8.75 mmol Ni2+ and 17.5 mmol Cl–
Total mmol Cl– after mixing = 25.0 + 17.5 = 42.5 mmol Cl–
Total volume after mixing = 50.0 mL + 25.0 mL = 75.0 mL
Mcobalt(II) ion =
2+
12.5 mmol Co
75.0 mL
= 0.167 M
Mnickel(II) ion =
2+
8.75 mmol Ni
75.0 mL
= 0.117 M
Mchloride ion =
42.5 mmol Cl
75.0 mL
= 0.567 M
92.
75 g solution
25 g NaCl
100. g solution
= 18.75 g NaCl
new % =
18.75 g NaCl
575 g solution
100 = 3.26 = 3.3 %
93.
AgNO3(s) + NaCl(aq) AgCl(s) + NaNO3(aq)
molar masses: AgNO3, 169.9 g; AgCl, 143.4 g
10.0 g AgNO3
3
3
1 mol AgNO
169.9 g AgNO
= 0.05886 mol AgNO3
50. mL = 0.050 L
0.050 L
2
1.0 10 mol NaCl
1.00 L
= 0.00050 mol NaCl
NaCl is the limiting reactant. 0.00050 mol AgCl form.
0.00050 mol AgCl
143.4 g AgCl
1 mol
= 0.072 g AgCl (72 mg)
As 1 mol AgNO3 contains 1 mol Ag+, the mol Ag+ remaining in solution = 0.05886 – 0.00050 =
0.05836 mol AgNO3.
0.05836 mol AgNO3 = 0.05836 mol Ag+
Msilver ion =
+
0.05836 mol Ag
0.050 L
= 1.167 M = 1.2 M
94.
molar mass NaHCO3 = 84.01 g; 25.2 mL = 0.0252 L
NaHCO3(s) + HCl(aq) NaCl(aq) + H2O(l)
336
Chapter 15: Solutions
mol HCl = mol NaHCO3 required = 0.0252 L ×
6.01 mol HCl
1.00 L
= 0.151 mol
0.151 mol ×
84.01 g
1 mol
= 12.7 g NaHCO3 required
95.
NiCl2(aq) + H2S(aq) NiS(s) + 2HCl(aq)
10. mL = 0.010 L
0.010 L ×
2
0.050 mol NiCl
1.00 L
= 0.00050 mol NiCl2
From the balanced equation, 0.00050 mol H2S will be required.
0.00050 mol H2S ×
22.4 L
1 mol
= 0.0112 L = 11 mL H2S
96.
molar mass H2O = 18.0 g
1.0 L water = 1.0 × 103 mL water 1.0 × 103 g water
1.0 × 103 g H2O ×
2
2
1 mol H O
18.0 g H O
= 56 mol H2O
97.
100. mL = 0.100 L
0.100 L ×
3
14.5 mol NH
1.00 L
= 1.45 mol NH3
1.45 mol NH3 ×
22.4 L
1 mol
= 32.5 L
98.
500 mL HCl solution = 0.500 L HCl solution
0.500 L solution ×
0.100 mol HCl
1.00 L solution
= 0.0500 mol HCl
0.0500 mol HCl ×
22.4 L
1 mol
= 1.12 L HCl gas at STP
99.
When we say “like dissolves like” we mean that two substances will be miscible if they have
similar intermolecular forces, so that the forces existing in the mixture will be similar to the
forces existing in each separate substance. Molecules do not have to be identical to be miscible,
but a similarity in structure (e.g., an OH group) will aid solubility.
100.
10.0 g HCl ×
100.g solution
33.1 g HCl
= 30.21 g solution
30.21 g solution ×
1.00 mL solution
1.147 g solution
= 26.3 mL solution
337

Chapter 15: Solutions
101.
1.00 g AgNO3 ×
3
100.0 g solution
0.50 g AgNO
= 200 g solution (2.0 × 102 mL solution)
102.
225.0 mL = 0.2250 L
150.0 mL = 0.1500 L
Determine the total number of moles of HCl in the solution and the total volume of solution.
Total number of moles of HCl = 0.56 mol + 0.11 mol = 0.67 mol HCl
Total volume of solution = 0.2250 L + 0.1500 L = 0.3750 L solution
103.
0.1 g CaCl2
104.
a.
x = 0.719 g NaCl
b.
x = 0.719 g NaCl
c.
x = 0.49 g NaCl
d.
x = 55.6 g NaCl
105.
To say a solution is 15.0% by mass NaCl means that 100.0 g of the solution would contain 15.0 g
of NaCl
a.
10.0 g NaCl ×
100. g solution
15.0 g NaCl
= 66.7 g solution
15.0 g NaCl
b.
25.0 g NaCl ×
100. g solution
15.0 g NaCl
= 167 g solution
c.
100.0 g NaCl ×
100. g solution
15.0 g NaCl
= 667 g solution
338
Chapter 15: Solutions
d.
1.00 lb = 453.59 g
453.59 g NaCl ×
100. g solution
15.0 g NaCl
= 3.02 × 103 g solution
106.
%C =
5.0 g C
(5.0 g C + 1.5 g Ni + 100. g Fe)
× 100 = 4.7% C
%Ni =
1.5 g Ni
(5.0 g C + 1.5 g Ni + 100. g Fe)
× 100 = 1.4% Ni
%Fe =
100. g Fe
(5.0 g C + 1.5 g Ni + 100. g Fe)
× 100 = 93.9% Fe
107.
25 g dextrose ×
100. g solution
10. g dextrose
= 250 g solution
108.
To say that the solution is 5.5% by mass Na2CO3 means that 5.5 g of Na2CO3 are contained in
every 100 g of the solution.
500. g solution ×
2
3
5.5 g Na CO
100. g solution
= 28 g Na2CO3
109.
125 g solution ×
3
1.5 g KNO
100. g solution
= 1.9 g KNO3
110.
For NaCl:
125 g solution ×
7.5 g NaCl
100. g solution
= 9.4 g NaCl
For KBr:
125 g solution ×
2.5 g KBr
100. g solution
= 3.1 g KBr
111.
0.0117 L ×
3
4
0.102 mol Na PO
1.00 L
= 1.19 × 10–3 mol Na3PO4
The sample would contain 1.19 × 10–3 mol of PO43– and 3(1.19 × 10–3) = 3.58 × 10–3 mol of Na+
ion.
112.
a.
250 mL = 0.25 L
b.
500. mL = 0.500 L
339

Chapter 15: Solutions
c.
750 mL = 0.75 L
d.
113.
a.
molar mass BaCl2 = 208.2 g
5.0 g BaCl2 ×
2
1 mol
208.2 g BaCl
= 0.0240 mol BaCl2
M =
2
0.240 mol BaCl
2.5 L solution
= 9.6 × 10–3 M
b.
molar mass KBr = 119.0 g; 75 mL = 0.075 L
3.5 g KBr ×
1 mol
119.0 g KBr
= 0.0294 mol KBr
M =
0.0294 mol KBr
0.075 L solution
= 0.39 M
c.
molar mass Na2CO3 = 106.0 g; 175 mL = 0.175 L
21.5 g Na2CO3 ×
2
3
1 mol
106.0 g Na CO
= 0.2028 mol Na2CO3
M =
2
3
0.2028 mol Na CO
0.175 L
= 1.16 M
d.
molar mass CaCl2 = 111.0 g
55 g CaCl2 ×
2
1 mol
111.0 g CaCl
= 0.495 mol CaCl2
M =
2
0.495 mol CaCl
1.2 L solution
= 0.41 M
114.
molar mass C12H22O11 = 342.3 g; 450. mL = 0.450 L
125 g C12H22O11 ×
1 mol
342.3 g
= 0.3652 mol C12H22O11
M =
0.3652 mol
0.450 L solution
= 0.812 M
115.
molar mass HCl = 36.46 g
439 g HCl ×
1 mol
36.46 g
= 12.04 mol HCl
340
Chapter 15: Solutions
M =
12.04 mol HCl
1.00 L solution
= 12.0 M
116.
a.
b.
The balanced equation is:
2FeCl3(aq) + 3Pb(NO3)2(aq) → 3PbCl2(s) + 2Fe(NO3)3(aq)
Determine the number of moles of each reactant present.
(already determined from part a)
Determine how many moles of PbCl2 (the solid) would be produced assuming each
reactant is completely consumed.
Once 2.00 mol PbCl2 is produced, all of the Pb2+ ion is used up (making Pb(NO3)2 the
limiting reactant). Thus,
117.
a.
1.5 L solution ×
2
4
3.0 mol H SO
1.00 L solution
= 4.5 mol H2SO4
b.
35 mL = 0.035 L
0.035 L solution ×
5.4 mol NaCl
1.00 L solution
= 0.19 mol NaCl
c.
5.2 L solution ×
2
4
18 mol H SO
1.00 L solution
= 94 mol H2SO4
d.
0.050 L ×
3
1.1 10 mol NaF
1.00 L solution
= 5.5 × 10–5 mol NaF
341
Chapter 15: Solutions
118.
a.
4.25 L solution ×
0.105 mol KCl
1.00 L solution
= 0.446 mol KCl
molar mass KCl = 74.6 g
0.446 mol KCl ×
74.6 g KCl
1 mol KCl
= 33.3 g KCl
b.
15.1 mL = 0.0151 L
0.0151 L solution ×
3
0.225 mol NaNO
1.00 L solution
= 3.40 × 10–3 mol NaNO3
molar mass NaNO3 = 85.00 g
3.40 × 10–3 mol ×
3
3
85.00 g NaNO
1 mol NaNO
= 0.289 g NaNO3
c.
25 mL = 0.025 L
0.025 L solution ×
3.0 mol HCl
1.00 L solution
= 0.075 mol HCl
molar mass HCl = 36.46 g
0.075 mol HCl ×
36.46 g HCl
1 mol HCl
= 2.7 g HCl
d.
100. mL = 0.100 L
0.100 L solution ×
2
4
0.505 mol H SO
1.00 L solution
= 0.0505 mol H2SO4
molar mass H2SO4 = 98.09 g
0.0505 mol H2SO4 ×
2
4
2
4
98.09 g H SO
1 mol H SO
= 4.95 g H2SO4
119.
molar mass AgNO3 = 169.9 g
10. g AgNO3 ×
3
3
1 mol AgNO
169.9 g AgNO
= 0.0589 mol AgNO3
0.0589 mol AgNO3 ×
3
1.00 L solution
0.25 mol AgNO
= 0.24 L solution
120.
a.
1.25 L ×
3
4
0.250 mol Na PO
1.00 L
= 0.3125 mol Na3PO4
0.3125 mol Na3PO4 ×
+
3
4
3 mol Na
1 mol Na PO
= 0.938 mol Na+
0.3125 mol Na3PO4 ×
4
3
4
1 mol PO
1 mol Na PO
= 0.313 mol PO43–
342
Chapter 15: Solutions
b.
3.5 mL = 0.0035 L
0.0035 L ×
2
4
6.0 mol H SO
1.00 L
= 0.021 mol H2SO4
0.021 mol H2SO4 ×
+
2
4
2 mol H
1 mol H SO
= 0.042 mol H+
0.021 mol H2SO4 ×
2
4
2
4
1 mol SO
1 mol H SO
= 0.021 mol SO42–
c.
25 mL = 0.025 L
0.025 L ×
3
0.15 mol AlCl
1.00 L
= 0.00375 mol AlCl3
0.00375 mol AlCl3 ×
3+
3
1 mol Al
1 mol AlCl
= 0.0038 mol Al3+
0.00375 mol AlCl3 ×
3
1 mol Cl
1 mol AlCl
= 0.011 mol Cl–
d.
1.50 L ×
2
1.25 mol BaCl
1.00 L
= 1.875 mol BaCl2
1.875 mol BaCl2 ×
2+
2
1 mol Ba
1 mol BaCl
= 1.88 mol Ba2+
1.875 mol BaCl2 ×
2
2 mol Cl
1 mol BaCl
= 3.75 mol Cl–
121.
molar mass CaCO3 = 100.1 g ; 500. mL = 0.500 L
0.500 L ×
3
0.0200 mol CaCO
1.00 L
= 0.0100 mol CaCO3 needed
0.0100 mol CaCO3 ×
3
3
100.1 g CaCO
1 mol CaCO
= 1.00 g CaCO3
122.
1
1
2
2
M
V
M
V
a.
M1 = 0.200 M
M2 = ?
V1 = 125 mL
V2 = 125 + 150. = 275 mL
M2 =
(0.200 )(125 mL)
(275 mL)
M
= 0.0909 M
b.
M1 = 0.250 M
M2 = ?
V1 = 155 mL
V2 = 155 + 150. = 305 mL
M2 =
(0.250 )(155 mL)
(305 mL)
M
= 0.127 M
343

Chapter 15: Solutions
c.
M1 = 0.250 M
M2 = ?
V1 = 0.500 L = 500. mL
V2 = 500. + 150. = 650. mL
M2 =
(0.250 )(500. mL)
(650 mL)
M
= 0.192 M
d.
M1 = 18.0 M
M2 = ?
V1 = 15 mL
V2 = 15 + 150. = 165 mL
M2 =
(18.0 )(15 mL)
(165 mL)
M
= 1.6 M
123.
1
1
2
2
M
V
M
V
M1 = 18.0 M
M2 = 0.250 M
V1 = ?
V2 = 35.0 mL
V1 =
(0.250 )(35.0 mL)
(18.0 mL)
M
= 0.486 mL
124.
1
1
2
2
M
V
M
V
M1 = 5.4 M
M2 = ?
V1 = 50. mL
V2 = 300. mL
M2 =
(5.4 )(50. mL)
(300. mL)
M
= 0.90 M
125.
1
1
2
2
M
V
M
V
M1 = 6.0 M
M2 = ?
V1 = 3.0 L
V2 = 10.0 + 3.0 = 13.0 L
M2 =
(6.0 )(3.0 L)
(13.0 L)
M
= 1.4 M
126.
a.
b.
The balanced equation is:
3Pb(NO3)2(aq) + 2Na3PO4(aq) → Pb3(PO4)2(s) + 6NaNO3(aq)
150.0 mL = 0.1500 L
0.250 L Na3PO4 = 250. mL Na3PO4
344
Chapter 15: Solutions
127.
15.3 mL = 0.0153 L; molar mass Ba(NO3)2 = 261.3 g
0.0153 L ×
2
4
0.139 mol H SO
1.00 L
= 2.127 × 10–3 mol H2SO4
2.127 × 10–3 mol H2SO4 ×
3 2
2
4
1 mol Ba(NO )
1 mol H SO
= 2.127 × 10–3 mol Ba(NO3)2
2.127 × 10–3 mol Ba(NO3)2 ×
3 2
3 2
261.3 g Ba(NO )
1 mol Ba(NO )
= 0.556 g Ba(NO3)2
128.
1
1
2
2
M
V
M
V
M1 = 16 M
M2 = 0.10 M
V1 = ?
V2 = 750 mL = 0.75 L
0.0047 L = 4.7 mL
129.
a.
HCl(aq) + NaOH(aq) NaCl(aq) + H2O(l)
25.0 mL = 0.0250 L
0.0250 L ×
0.103 mol NaOH
1.00 L
= 0.02575 mol NaOH
0.02575 mol NaOH ×
1 mol HCl
1 mol NaOH
= 0.02575 mol HCl
0.02575 mol HCl ×
1.00 L
0.250 mol HCl
= 0.0103 L HCl = 10.3 mL HCl
b.
2HCl(aq) + Ca(OH)2(aq) CaCl2(aq) + 2H2O(l)
50.0 mL = 0.0500 L
0.0500 L ×
2
0.00501 mol Ca(OH)
1.00 L
= 2.505 × 10–4 mol Ca(OH)2
2.505 × 10–4 mol Ca(OH)2 ×
2
2 mol HCl
1 mol Ca(OH)
= 5.010 × 10–4 mol HCl
5.010 × 10–4 mol HCl ×
1.00 L
0.250 mol HCl
= 0.00200 L = 2.00 mL
c.
HCl(aq) + NH3(aq) NH4Cl(aq)
20.0 mL = 0.0200 L
0.0200 L ×
3
0.226 mol NH
1.00 L
= 0.00452 mol NH3
345
Chapter 15: Solutions
0.00452 mol NH3 ×
3
1 mol HCl
1 mol NH
= 0.00452 mol HCl
0.00452 mol HCl ×
1.00 L
0.250 mol HCl
= 0.01808 L = 18.1 mL
d.
HCl(aq) + KOH(aq) KCl(aq) + H2O(l)
15.0 mL = 0.0150 L
0.0150 L ×
0.0991 mol KOH
1.00 L
= 1.487 × 10–3 mol KOH
1.487 × 10–3 mol KOH ×
1 mol HCl
1 mol KOH
= 1.487 × 10–3 mol HCl
1.487 × 10–3 mol HCl ×
1.00 L
0.250 mol HCl
= 0.00595 L = 5.95 mL
130.
a.
equivalent weight HCl = molar mass HCl = 36.46 g; 500. mL = 0.500 L
15.0 g HCl ×
1 equiv HCl
36.46 g HCl
= 0.411 equiv HCl
N =
0.411 equiv
0.500 L
= 0.822 N
b.
equivalent weight H2SO4 =
molar mass
98.09 g
2
2
= 49.05 g; 250. mL = 0.250 L
49.0 g H2SO4 ×
2
4
2
4
1 equiv H SO
49.05 g H SO
= 0.999 equiv H2SO4
N =
0.999 equiv
0.250 L
= 4.00 N
c.
equivalent weight H3PO4 =
molar mass
98.0 g
3
3
= 32.67 g; 100. mL = 0.100 L
10.0 g H3PO4 ×
3
4
3
4
1 equiv H PO
32.67 g H PO
= 0.3061 equiv H3PO4
N =
0.3061 equiv
0.100 L
= 3.06 N
131.
a.
0.50 M HC2H3O2 ×
2
3
2
2
3
2
1 equiv HC H O
1 mol HC H O
= 0.50 N HC2H3O2
b.
0.00250 M H2SO4 ×
2
4
2
4
2 equiv H SO
1 mol H SO
= 0.00500 N H2SO4
c.
0.10 M KOH ×
1 equiv KOH
1 mol KOH
= 0.10 N KOH
346

Chapter 15: Solutions
132.
molar mass NaH2PO4 = 120.0 g; 500. mL = 0.500 L
5.0 g NaH2PO4 ×
2
4
2
4
1 mol NaH PO
120.0 g NaH PO
= 0.04167 mol NaH2PO4
M =
0.04167 mol
0.500 L
= 0.08333 M NaH2PO4 = 0.083 M NaH2PO4
0.08333 M NaH2PO4 ×
2
4
2
4
2 equiv NaH PO
1 mol NaH PO
= 0.1667 N NaH2PO4 = 0.17 N NaH2PO4
133.
3NaOH(aq) + H3PO4(aq) Na3PO4(aq) + 3H2O(l)
14.2 mL = 0.0142 L
0.0142 L ×
3
4
0.141 mol H PO
1.00 L
= 2.00 × 10–3 mol H3PO4
2.00 × 10–3 mol H3PO4 ×
3
4
3 mol NaOH
1 mol H PO
= 6.00 × 10–3 mol NaOH
6.00 × 10–3 mol NaOH ×
1.00 L
0.105 mol NaOH
= 5.72 × 10–2 L = 57.2 mL
134.
Nacid × Vacid = Nbase × Vbase
Nacid × (10.0 mL) = (3.5 × 10–2 N)(27.5 mL)
Nacid = 9.6 × 10–2 N HNO3
135.
molar mass CsCl = 168.35 g; molar mass Na2SO4 = 142.05 g; molar mass CsF = 151.9 g; molar
mass Na3PO4 = 163.94 g
Therefore, Solution #2 has the highest concentration.
136.
The solution with the greatest number of ions will have the greatest number of moles of ions.
Soln #1: CsCl(aq) → Cs+(aq) + Cl–(aq); 1 mol CsCl produces 2 moles of ions.
347

Chapter 15: Solutions
Soln #2: Na2SO4(aq) → 2Na+(aq) + SO42–(aq); 1 mol Na2SO4 produces 3 moles of ions.
Soln #3: CsF(aq) → Cs+(aq) + F–(aq); 1 mol CsF produces 2 moles of ions.
Soln #4: Na3PO4(aq) → 3Na+(aq) + PO43–(aq); 1 mol Na3PO4 produces 4 moles of ions.
Therefore, Solution #4 contains the greatest number of ions.
137.
100.0 mL = 0.1000 L
molar mass MgCl2 = 95.21 g
138.
molar mass H2C2O4 = 90.036 g
100.0 mL = 0.1000 L; 10.00 mL = 0.01000 L; 250.0 mL = 0.2500 L
139.
150.0 mL = 0.1500 L
The balanced equation is: 2NaOH(aq) + Ni(NO3)2(aq) Ni(OH)2(s) + 2NaNO3(aq)
348

Chapter 15: Solutions
0.747 L = 747 mL NaOH
140.
500.0 mL = 0.5000 L; 400.0 mL = 0.4000 L
molar mass Ba3(PO4)2 = 601.84 g
The balanced equation is: 2Na3PO4(aq) + 3BaCl2(aq) Ba3(PO4)2(s) + 6NaCl(aq)
BaCl2 is the limiting reactant (only 0.0771 mol Na3PO4 needed).
(when decimals carried over)
141.
450.0 mL = 0.4500 L; 400.0 mL = 0.4000 L
The balanced equation is: 2AgNO3(aq) + CaCl2(aq) 2AgCl(s) + Ca(NO3)2(aq)
AgNO3 is the limiting reactant (only 0.0578 mol CaCl2 needed).
0.0800 mol CaCl2 to start – 0.0578 mol CaCl2 used up = 0.0222 mol CaCl2 leftover
CaCl2(aq) Ca2+(aq) + 2Cl–(aq)
(when decimals carried over)
349

Chapter 15: Solutions
142.
34.66 mL = 0.03466 L; 50.00 mL = 0.05000 L
The balanced equation is: Ca(OH)2(aq) + 2HNO3(aq) Ca(NO3)2(aq) + 2H2O(l)
(when decimals carried over)
143.
28.44 mL = 0.02844 L
The balanced equations are:
H2O2 + SO2 H2SO4
H2SO4 + 2NaOH Na2SO4 + 2H2O
(when decimals carried over)
350