
CHAPTER 18
Oxidation–Reduction Reactions:
Electrochemistry
1.
Oxidation reduction reactions are involved in electrical batteries, corrosion and its prevention, co
mbustion of fuels, many cleaning agents, and metabolic processes, to name a few.
2.
Oxidation can be defined as the loss of electrons by an atom, molecule, or ion. Oxidation may als
o be defined as an increase in oxidation state for an element, but because elements can only increa
se their oxidation states by losing electrons, the two definitions are equivalent. The following equ
ation shows the oxidation of copper metal to copper(II) ion
Cu Cu2+ + 2e–
Reduction can be defined as the gaining of electrons by an atom, molecule, or ion. Reduction may
also be defined as a decrease in oxidation state for an element, but naturally such a decrease takes
place by the gaining of electrons (so the two definitions are equivalent). The following equation
shows the reduction of sulfur atoms to sulfide ion.
S + 2e– S2–
3.
Each of these reactions involves one or more free elements on one side of the equation; on the oth
er side of the equation, however, the element(s) is(are) combined in a compound. This is a clear si
gn that an oxidation–reduction process is taking place.
a.
chlorine is reduced; iodine is oxidized
b.
chlorine is reduced, lithium is oxidized
c.
sodium is oxidized; hydrogen is reduced
d.
bromine is oxidized; chlorine is reduced
4.
Each of these reactions involves one or more free elements on one side of the equation; on the oth
er side of the equation, however, the element(s) is(are) combined in a compound. This is a clear si
gn that an oxidation–reduction process is taking place.
a.
sodium is oxidized; nitrogen is reduced
b.
magnesium is oxidized; chlorine is reduced
c.
aluminum is oxidized; bromine is reduced
d.
magnesium is oxidized; copper is reduced
5.
Each of these reactions involves one or more free elements on one side of the equation; on the oth
er side of the equation, however, the element(s) is(are) combined in a compound. This is a clear si
gn that an oxidation–reduction process is taking place.
a.
calcium is oxidized; hydrogen is reduced
b.
hydrogen is oxidized; fluorine is reduced
406

Chapter 18: Oxidation–Reduction Reactions/Electrochemistry
c.
iron is oxidized; oxygen is reduced
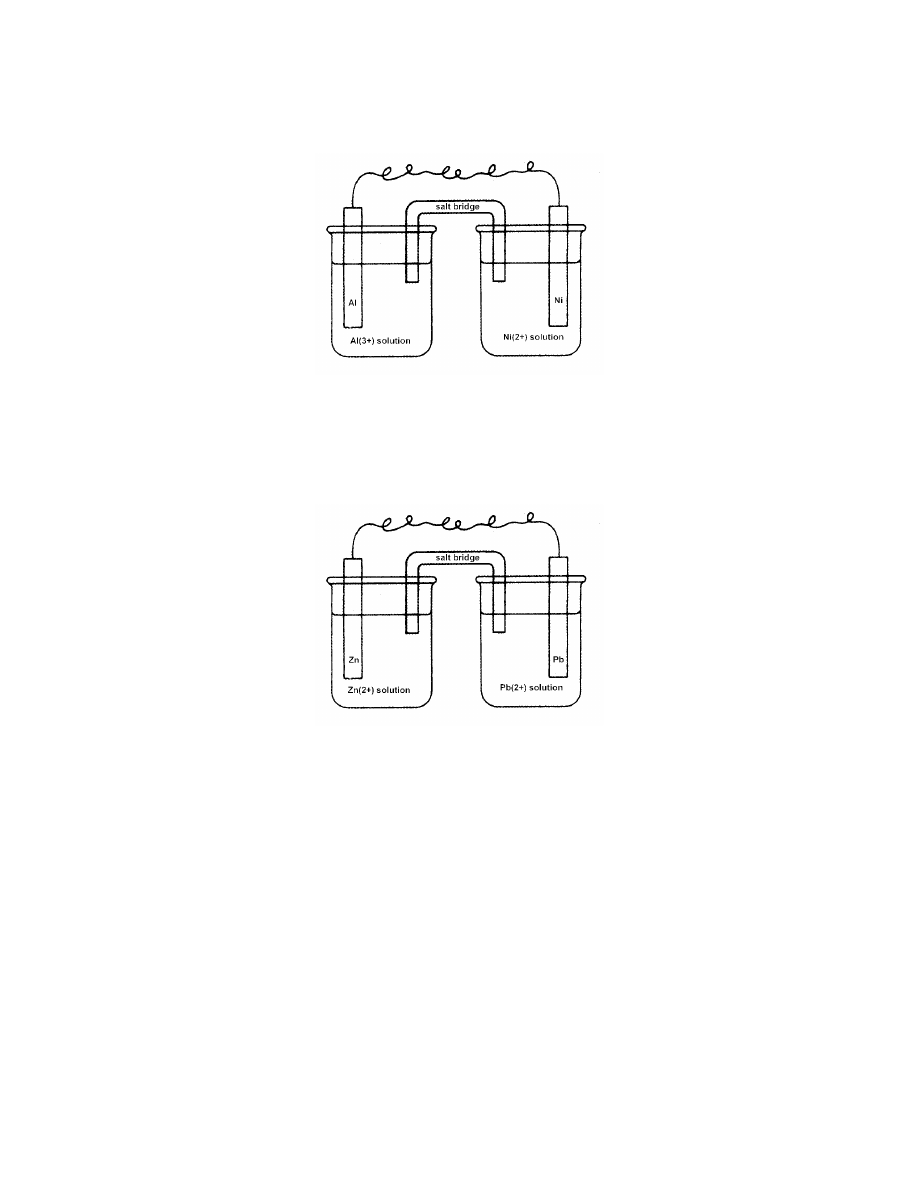
d.
iron is oxidized; chlorine is reduced
6.
Each of these reactions involves one or more free elements on one side of the equation; on the oth
er side of the equation, however, the element(s) is(are) combined in a compound. This is a clear si
gn that an oxidation–reduction process is taking place.
a.
magnesium is oxidized; bromine is reduced
b.
sodium is oxidized; sulfur is reduced
c.
hydrogen is oxidized; carbon is reduced
d.
potassium is oxidized; nitrogen is reduced
7.
The assignment of oxidation states is a bookkeeping method. Charges are assigned to the various
atoms in a compound; this method allows us to keep track of electrons transferred between specie
s in oxidation– reduction reactions.
8.
A neutral molecule has an overall charge of zero.
9.
For the very electronegative elements (such as oxygen), we assign an oxidation state equal to its c
harge when the element forms an anion. As oxygen forms O2– ions, we assign oxygen an oxidatio
n state of –2 even in compounds where no ions exist. The most common situation in which oxyge
n is not assigned the –2 oxidation state occurs with the peroxide ion, O22–, as each oxygen atom is
in the –1 oxidation state.
10.
Fluorine is always assigned a negative oxidation state (–1) because all other elements are less elec
tronegative. The other halogens are usually assigned an oxidation state of –1 in compounds. In int
erhalogen compounds such as ClF, fluorine is assigned oxidation state –1 (F is more electronegati
ve than Cl). Chlorine, therefore, must be assigned a +1 oxidation state in this instance.
11.
Oxidation states represent a bookkeeping method to assign electrons in a molecule or ion. As a ne
utral molecule has an overall charge of zero, the sum of the oxidation states in a neutral molecule
must be zero. So in H3PO4, the sum of all the oxidation states must be zero.
12.
The sum of all the oxidation states of the atoms in a polyatomic ion must equal the overall charge
on the ion. The sum of all the oxidation states of all the atoms in PO43– is –3.
13.
The rules for assigning oxidation states are given in Section 18.2 of the text. The rule that applies
for each element in the following answers is given in parentheses after the element and its oxidati
on state.
a.
C, +4 (Rule 6); Br, –1 (Rule 5)
b.
H, +1 (Rule 4); Cl, +7 (Rule 6); O, –2 (Rule 3)
c.
K, +1 (Rule 2); P, +5 (Rule 6); O, –2 (Rule 3)
d.
N, +1 (Rule 6); O, –2 (Rule 3)
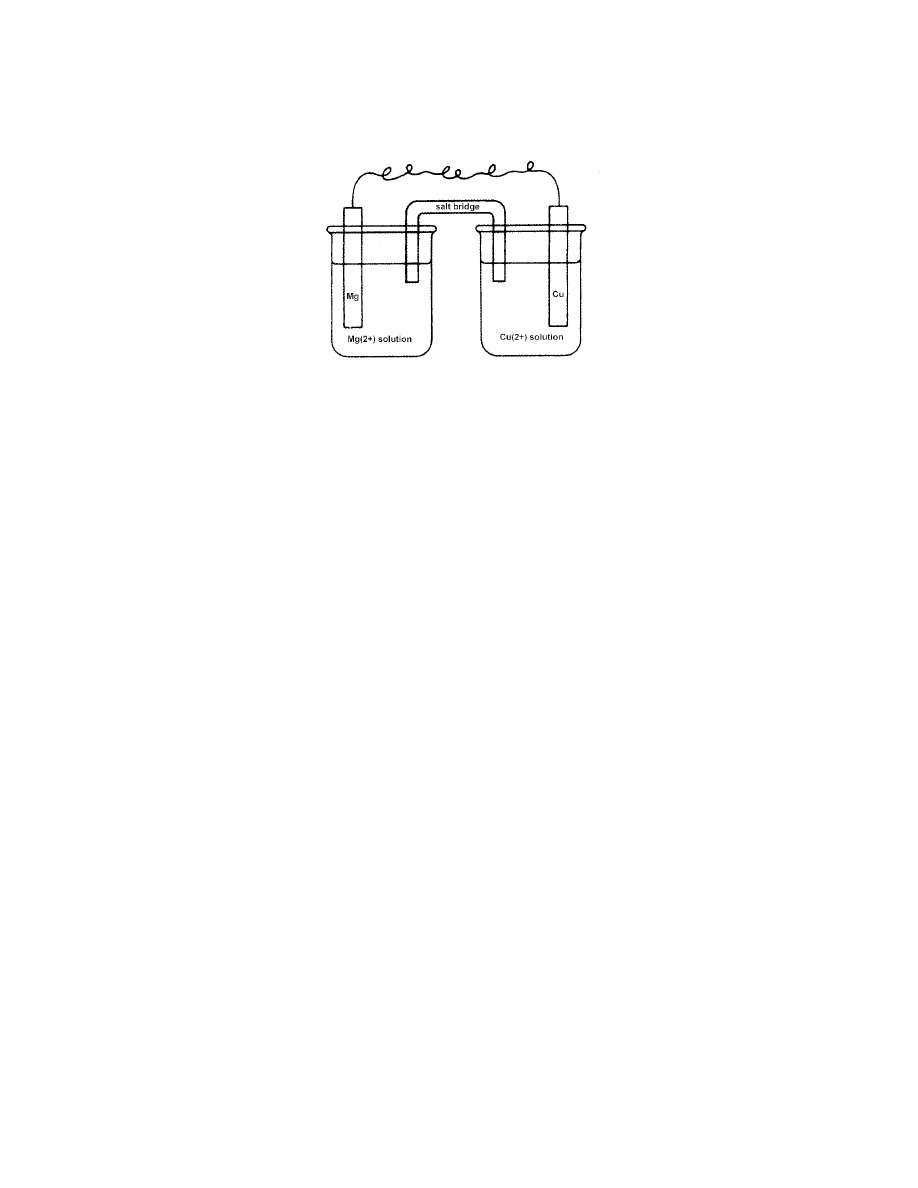
14.
The rules for assigning oxidation states are given in Section 18.2 of the text. The rule that applies
for each element in the following answers is given in parentheses after the element and its oxidati
on state.
407

Chapter 18: Oxidation–Reduction Reactions/Electrochemistry
a.
N, +3 (Rule 6); Cl, –1 (Rule 5)
b.
S, +6 (Rule 6); F, –1 (Rule 5)
c.
P, +5 (Rule 6); Cl, –1 (Rule 5)
d.
Si, –4 (Rule 6); H, +1 (Rule 4)
15.
The rules for assigning oxidation states are given in Section 18.2 of the text. The rule that applies
for each element in the following answers is given in parentheses after the element and its oxidati
on state.
a.
0 (Rule 1)
b.
+6 (Rule 6)
c.
+6 (Rule 6)
d.
–2 (Rule 5)
16.
a.
0 (Rule 1)
b.
–3 (using Rule 4 for H)
c.
+4 (using Rule 3 for O)
d.
+5 (using Rule 3 for O and Rule 2 for Na)
17.
a.
+1 (using Rule 5 for F)
b.
0 (Rule 1)
c.
–1 (Rule 5)
d.
+1 (using Rule 3 for O and Rule 4 for H)
18.
a.
+2 (using Rule 5 for Cl)
b.
+7 (using Rule 3 for O and Rule 2 for K)
c.
+4 (using Rule 3 for O)
d.
+3 (realizing that the acetate ion has 1– charge and apply Rule 6)
19.
The rules for assigning oxidation states are given in Section 18.2 of the text. The rule that applies
for each element in the following answers is given in parentheses after the element and its oxidati
on state.
a.
Cu, +2 (Rule 6); Cl, –1 (Rule 5)
b.
K, +1 (Rule 2); Cl, +5 (Rule 6); O, –2 (Rule 3)
c.
K, +1 (Rule 2); Cl, +7 (Rule 6); O, –2 (Rule 3)
d.
Na, +1 (Rule 2); C, +4 (Rule 6); O, –2 (Rule 3)
20.
The rules for assigning oxidation states are given in Section 18.2 of the text. The rule that applies
for each element in the following answers is given in parentheses after the element and its oxidati
on state.
a.
Ca, +2 (Rule 6); O, –2 (Rule 3)
b.
Al, +3 (Rules 6 and 2); O, –2 (Rule 3)
408

Chapter 18: Oxidation–Reduction Reactions/Electrochemistry
c.
P, +3 (Rule 6); F, –1 (Rule 5)
d.
P, +5 (Rule 6); O, –2 (Rule 3)
21.
The rules for assigning oxidation states are given in Section 18.2 of the text. The rule that applies
for each element in the following answers is given in parentheses after the element and its oxidati
on state.
a.
C, +4 (Rule 7); O, –2 (Rule 3)
b.
N, +5 (Rule 7); O, –2 (Rule 3)
c.
P, +5 (Rule 7); O, –2 (Rule 3)
d.
S, +6 (Rule 7); O, –2 (Rule 3)
22.
The rules for assigning oxidation states are given in Section 18.2 of the text. The rule that applies
for each element in the following answers is given in parentheses after the element and its oxidati
on state.
a.
H, +1 (Rule 4); S, +6 (Rule 7); O, –2 (Rule 3)
b.
Mn, +7 (Rule 7); O, –2 (Rule 3)
c.
Cl, +5 (Rule 7); O, –2 (Rule 3)
d.
Br, +7 (Rule 7; O, –2 (Rule 3)
23.
Consider the following simple oxidation reaction
Na Na+ + e–
Clearly the sodium atom on the left side of the equation is losing an electron in forming the
sodium ion on the right side of the equation. The sodium atom on the left side of the equation is
in the zero oxidation state because it represents a pure element. The sodium ion on the right side
of the equation is in the +1 oxidation state (the same as the charge on the simple ion). Thus, by
losing one electron, sodium has increased in oxidation state by one unit.
24.
Electrons are negative; when an atom gains electrons, it gains one negative charge for each electr
on gained. For example, in the reduction reaction Cl + e– Cl–, the oxidation state of chlorine d
ecreases from 0 to –1 as the electron is gained.
25.
An oxidizing agent is a molecule, atom, or ion that causes the oxidation of some other species, w
hile itself being reduced. A reducing agent is a molecule, atom, or ion that causes the reduction of
some other species, while itself being oxidized.
26.
An oxidizing agent decreases its oxidation state. A reducing agent increases its oxidation state.
27.
Oxidizing agents gain the electrons they cause other species to lose. Reducing agents donate the
electrons needed for the reduction of some other species.
28.
An antioxidant is a substance that prevents oxidation of some molecule(s) in the body. It is not ce
rtain how all antioxidants work, but one example is in preventing oxygen molecules and other sub
stances from stripping electrons from cell membranes, leaving them vulnerable to destruction by t
he immune system.
409

Chapter 18: Oxidation–Reduction Reactions/Electrochemistry
29.
a.
Fe(s) + CuSO4(aq) FeSO4(aq) + Cu(s)
iron is being oxidized, copper is being reduced
b.
Cl2(g) + 2NaBr(aq) 2NaCl(aq) + Br2(l)
bromine is being oxidized, chlorine is being reduced
c.
3CuS(s) + 8HNO3(aq) 3CuSO4(aq) + 8NO(g) + 4H2O(l)
sulfur is being oxidized, nitrogen is being reduced
d.
2Zn(s) + O2(g) 2ZnO(s)
zinc is being oxidized, oxygen is being reduced
30. a.
2Al(s) + 3S(s) Al2S3(s)
aluminum is being oxidized, sulfur is being reduced
b.
CH4(g) + 2O2(g) CO2(g) + 2H2O(g)
carbon is being oxidized, oxygen is being reduced
c.
2Fe2O3(s) + 3C(s) 3CO2(g) + 4Fe(s, l)
carbon is being oxidized, iron is being reduced
d.
K2Cr2O7(aq) + 14HCl(aq) 2KCl(aq) + 2CrCl3(s) + 7H2O(l) + 3Cl2(g)
chlorine is being oxidized, chromium is being reduced
31.
a.
2Cu(s) + S(s) Cu2S
copper is being oxidized; sulfur is being reduced
b.
2Cu2O(s) + O2(g) 4CuO(s)
copper is being oxidized; oxygen (of O2) is being reduced
c.
4B(s) + 3O2(g) 2B2O3(s)
boron is being oxidized; oxygen is being reduced
d.
6Na(s) + N2(g) 2Na3N(s)
sodium is being oxidized; nitrogen is being reduced
32.
a.
4KClO3(s) + C6H12O6(s) 4KCl(s) + 6H2O(l) + 6CO2(g)
carbon is being oxidized, chlorine is being reduced
b.
2C8H18(l) + 25O2(g) 16CO2(g) + 18H2O(l)
carbon is being oxidized, oxygen is being reduced
c.
PCl3(g) + Cl2(g) PCl5(g)
phosphorus is being oxidized, chlorine is being reduced
d.
Ca(s) + H2(g) CaH2(g)
calcium is being oxidized, hydrogen is being reduced
33.
Zinc is oxidized [0 in Zn(s), +2 in ZnCl2(aq)]; hydrogen is reduced [+1 in HCl(aq), 0 in H2(g)].
410

Chapter 18: Oxidation–Reduction Reactions/Electrochemistry
34.
Iron is reduced [+3 in Fe2O3(s), 0 in Fe(l)]; carbon is oxidized [+2 in CO(g), +4 in CO2(g)]. Fe2O3
(s) is the oxidizing agent; CO(g) is the reducing agent.
35.
Magnesium is oxidized [0 in Mg(s), +2 in Mg(OH)2(s)]; hydrogen is reduced [+1 in H2O(l), 0 in
H2(g)].
36.
a.
chlorine is being reduced, iodine is being oxidized; chlorine is the oxidizing agent, iodide
ion is the reducing agent
b.
iron is being reduced, iodine is being oxidized; iron(III) is the oxidizing agent, iodide ion
is the reducing agent
c.
copper is being reduced, iodine is being oxidized; copper(II) is the oxidizing agent,
iodide ion is the reducing agent
37.
Oxidation–reduction reactions must be balanced with respect to mass (the total number of each ty
pe of atom on each side of the balanced equation must be the same); with respect to charge, what
ever number of electrons is lost in the oxidation process must be gained in the reduction process,
with no “extra” electrons.
38.
Oxidation–reduction reactions are often more complicated than “regular” reactions; frequently the
coefficients necessary to balance the number of electrons transferred come out to be large number
s. We also have to make certain that we account for the electrons being transferred.
39.
When an overall equation is split into separate partial equations representing the oxidation and th
e reduction processes, these partial equations are called half–reactions.
40.
Under ordinary conditions it is impossible to have “free” electrons that are not part of some atom,
ion, or molecule. For this reason, the total number of electrons lost by the species being oxidized
must equal the total number of electrons gained by the species being reduced.
41.
a.
Cu Cu2+
Balance charge: Cu Cu2+ + 2e–
Balanced half–reaction: Cu Cu2+ + 2e–
b.
Fe3+ Fe2+
Balance charge: e– + Fe3+ Fe2+
Balanced half–reaction: e– + Fe3+ Fe2+
c.
Br– Br2
Balance mass: 2Br– Br2
Balance charge: 2Br– Br2 + 2e–
Balanced half–reaction: 2Br– Br2 + 2e–
d.
Fe2+ Fe
Balance charge: Fe2+ + 2e– Fe
Balanced half–reaction: Fe2+ + 2e– Fe
42.
a.
N2(g) N3–(aq)
411

Chapter 18: Oxidation–Reduction Reactions/Electrochemistry
balance nitrogen: 3N2(g) 2N3–(aq)
balance charge: 3N2(g) + 2e– 2N3–(aq)
balanced half-reaction: 3N2(g) + 2e– 2N3–(aq)
b.
O22–(aq) O2(g)
balance charge: O22–(aq) O2(g) + 2e–
balanced half-reaction: O22–(aq) O2(g) + 2e–
c.
Zn(s) Zn2+(aq)
balance charge: Zn(s) Zn2+(aq) + 2e–
balanced half-reaction: Zn(s) Zn2+(aq) + 2e–
d.
F2(g) F–(aq)
balance flourine: F2(g) 2F–(aq)
balance charge: F2(g) + 2e– 2F–(aq)
balanced half-reaction: F2(g) + 2e– 2F–(aq)
43.
a.
HClO(aq) Cl–(aq)
Balance oxygen: HClO Cl– + H2O
Balance hydrogen: H+ + HClO Cl– + H2O
Balance charge: 2e– + H+ + HClO Cl– + H2O
Balanced half–reaction: 2e– + H+(aq) + HClO(aq) Cl–(aq) + H2O(l)
b.
NO(aq) N2O(g)
Balance nitrogen: 2NO N2O
Balance oxygen: 2NO N2O + H2O
Balance hydrogen: 2H+ + 2NO N2O + H2O
Balance charge: 2e– + 2H+ + 2NO N2O + H2O
Balanced half–reaction: 2e– + 2H+(aq) + 2NO(aq) N2O(g) + H2O(l)
c.
N2O(aq) N2(g)
Balance oxygen: N2O N2 + H2O
Balance hydrogen: 2H+ + N2O N2 + H2O
Balance charge: 2e– + 2H+ + N2O N2 + H2O
Balanced half–reaction: 2e– + 2H+(aq) + N2O(aq) N2(g) + H2O(l)
d.
ClO3–(aq) HClO2(l)
Balance oxygen: ClO3– HClO2 + H2O
Balance hydrogen: 3H+ + ClO3– HClO2 + H2O
Balance charge: 2e– + 3H+ + ClO3– HClO2 + H2O
Balanced half–reaction: 2e– + 3H+(aq) + ClO3–(aq) HClO2(aq) + H2O(l)
412

Chapter 18: Oxidation–Reduction Reactions/Electrochemistry
44.
a.
O2(g) H2O(l)
balance oxygen: O2 2H2O
balance hydrogen: 4H+ + O2 2H2O
balance charge: 4e– + 4H+ + O2 2H2O
balanced half-reaction: 4e– + 4H+(aq) + O2(g) 2H2O(l)
b.
SO42–(aq) H2SO3(aq)
balance oxygen: SO42– H2SO3 + H2O
balance hydrogen: 4H+ + SO42– H2SO3 + H2O
balance charge: 2e– + 4H+ + SO42– H2SO3 + H2O
balanced half-reaction: 2e– + 4H+(aq) + SO42–(aq) H2SO3(aq) + H2O(l)
c.
H2O2(aq) H2O(l)
balance oxygen : H2O2 2H2O
balance hydrogen : 2H+ + H2O2 2H2O
balance charge : 2e– + 2H+ + H2O2 2H2O
balanced half-reaction: 2e– + 2H+(aq) + H2O2(aq) 2H2O(l)
d.
NO2–(aq) NO3–(aq)
balance oxygen : H2O + NO2– NO3–
balance hydrogen: H2O + NO2– NO3– + 2H+
balance charge: H2O + NO2– NO3– + 2H+ + 2e–
balanced half-reaction: H2O(l) + NO2–(aq) NO3–(aq) + 2H+(aq) + 2e–
45.
a.
Mg(s) + Hg2+(aq) Mg2+(aq) + Hg22+(aq)
Mg Mg2+
Balance charge: Mg Mg2+ + 2e–
Balanced half–reaction: Mg Mg2+ + 2e–
Hg2+ Hg22+
Balance mercury: 2Hg2+ Hg22+
Balance charge: 2Hg2+ + 2e– Hg22+
Balanced half–reaction: 2Hg2+ + 2e– Hg22+
As the number of electrons is the same in both half–reactions, the half–reactions can be
directly combined for the overall equation.
Mg(s) + 2Hg2+(aq) Mg2+(aq) + Hg22+(aq).
413

Chapter 18: Oxidation–Reduction Reactions/Electrochemistry
b.
NO3–(aq) + Br–(aq) NO(g) + Br2(l)
2NO3– NO
Balance oxygen: NO3– NO + 2H2O
Balance hydrogen: 4H+ + NO3– NO + 2H2O
Balance charge: 4H+ + NO3– + 3e– NO + 2H2O
Balanced half–reaction: 4H+ + NO3– + 3e– NO + 2H2O
Br– Br2
Balance bromine: 2Br– Br2
Balance charge: 2Br– Br2 + 2e–
Balanced half–reaction: 2Br– Br2 + 2e–
Combine the half–reactions:
2 (4H+ + NO3– + 3e– NO + 2H2O)
3 (2Br– Br2 + 2e–)
8H+(aq) + 2NO3–(aq) + 6Br–(aq) 3Br2(l) + 2NO(g) + 4H2O(l)
c.
Ni(s) + NO3–(aq) Ni2+(aq) + NO2(g)
Ni Ni2+
Balance charge: Ni Ni2+ + 2e–
Balanced half–reaction: Ni Ni2+ + 2e–
NO3– NO2
Balance oxygen: NO3– NO2 + H2O
Balance hydrogen: NO3– + 2H+ NO2 + H2O
Balance charge: NO3– + 2H+ + e– NO2 + H2O
Balanced half–reaction: NO3– + 2H+ + e– NO2 + H2O
Combine the half–reactions:
2 × (NO3– + 2H+ + e– NO2 + H2O)
Ni Ni2+ + 2e–
2NO3–(aq) + 4H+(aq) + Ni(s) 2NO2(g) + 2H2O(l) + Ni2+(aq)
d.
ClO4–(aq) + Cl–(aq) ClO3–(aq) + Cl2(g)
ClO4– ClO3–
Balance oxygen: ClO4– ClO3– + H2O
Balance hydrogen: 2H+ + ClO4– ClO3– + H2O
Balance charge: 2H+ + ClO4– + 2e– ClO3– + H2O
Balanced half–reaction: 2H+ + ClO4– + 2e– ClO3– + H2O
Cl– Cl2
Balance chlorine: 2Cl– Cl2
414

Chapter 18: Oxidation–Reduction Reactions/Electrochemistry
Balance charge: 2Cl– Cl2 + 2e–
Balanced half–reaction: 2Cl– Cl2 + 2 e–
Since the number of electrons transferred is the same in both half–reactions, the half–
reactions can be combined directly to give the overall equation for the reaction:
2H+(aq) + ClO4–(aq) + 2Cl–(aq) ClO3–(aq) + H2O(l) + Cl2(g)
46.
For simplicity, the physical states of the substances have been omitted until the final balanced equ
ation is given.
a.
Al(s) + H+(aq) Al3+(aq) + H2(g)
Al Al3+
Balance charge: Al Al3+ + 3e–
H+ H2
Balance hydrogen: 2H+ H2
Balance charge: 2e– + 2H+ H2
Combine half–reactions:
3 × (2e– + 2H+ H2)
2 × (Al Al3+ + 3e–)
2Al(s) + 6H+(aq) 2Al3+(aq) + 3H2(g)
b.
S2–(aq) + NO3–(g) S(s) + NO(g)
S2– S
Balance charge: S2– S + 2e–
NO3– NO
Balance oxygen: NO3– NO + 2H2O
Balance hydrogen: 4H+ + NO3– NO + 2H2O
Balance charge: 3e– + 4H+ + NO3– NO + 2H2O
Combine half–reactions:
3 × (S2– S + 2e–)
2 × (3e– + 4H+ + NO3– NO + 2H2O)
8H+ + 3S2–(aq) + 2NO3–(g) 3S(s) + 2NO(g) + 4H2O
c.
I2(aq) + Cl2(aq) IO3–(aq) + HCl(g)
I2 IO3–
Balance iodine: I2 2IO3–
Balance oxygen: 6H2O + I2 2IO3–
Balance hydrogen: 6H2O + I2 2IO3– + 12H+
Balance charge: 6H2O + I2 2IO3– + 12H+ + 10e–
Cl2 HCl
415

Chapter 18: Oxidation–Reduction Reactions/Electrochemistry
Balance chlorine: Cl2 2HCl
Balance hydrogen: 2H+ + Cl2 2HCl
Balance charge: 2e– + 2H+ + Cl2 2HCl
Combine half–reactions:
5 × (2e– + 2H+ + Cl2 2HCl)
6H2O + I2 2IO3– + 12H+ + 10e–
6H2O(l) + 2I2(aq) + 5Cl2(aq) 2IO3–(aq) + 10HCl(g) + 2H+(aq)
d.
AsO4–(aq) + S2–(aq) AsO3–(s) + S(s)
AsO4– AsO3–
Balance oxygen: AsO4– AsO3– + H2O
Balance hydrogen: 2H+ + AsO4– AsO3– + H2O
Balance charge: 2e– + 2H+ + AsO4– AsO3– + H2O
S2– S
Balance charge: S2– S + 2e–
2H+(aq) + AsO4–(aq) + S2–(aq) AsO3–(s) + S(s) + H2O(l)
47.
For simplicity, the physical states of the substances have been omitted until the final balanced equ
ation is given.
For the oxidation of iodide ion, I–, in acidic solution, the half–reaction is always the same:
I– I2
Balance iodine: 2I– I2
Balance charge: 2I– I2 + 2e–
Balanced half–reaction: 2I– I2 + 2e–
a.
IO3– I2
Balance iodine: 2IO3– I2
Balance oxygen: 2IO3– I2 + 6H2O
Balance hydrogen: 2IO3– + 12H+ I2 + 6H2O
Balance charge: 2IO3– + 12H+ + 10e– I2 + 6H2O
Balanced half–reaction: 2IO3– + 12H+ + 10e– I2 + 6H2O
Combine the half–reactions:
2IO3– + 12H+ + 10e– I2 + 6H2O
5 × (2I– I2 + 2e–)
2IO3–(aq) + 12H+(aq) + 10I–(aq) 6I2(aq) + 6H2O(l)
IO3–(aq) + 6H+(aq) + 5I–(aq) 3I2(aq) + 3H2O(l)
b.
Cr2O72– Cr3+
Balance chromium: Cr2O72– 2Cr3+
416

Chapter 18: Oxidation–Reduction Reactions/Electrochemistry
Balance oxygen: Cr2O72– 2Cr3+ + 7H2O
Balance hydrogen: Cr2O72– + 14H+ 2Cr3+ + 7H2O
Balance charge: Cr2O72– + 14H+ + 6e– 2Cr3+ + 7H2O
Balanced half–reaction: Cr2O72– + 14H+ + 6e– 2Cr3+ + 7H2O
Combine the half–reactions:
3 × (2I– I2 + 2e–)
Cr2O72– + 14H+ + 6e– 2Cr3+ + 7H2O
6I–(aq) + Cr2O72–(aq) + 14H+(aq) 3I2(aq) + 2Cr3+(aq) + 7H2O(l)
c.
Cu2+ CuI
Balance iodine: Cu2+ + I– CuI
Balance charge: Cu2+ + I– + e– CuI
Balanced half–reaction: Cu2+ + I– + e– CuI
Combine the half–reactions:
2I– I2 + 2e–
2 × (Cu2+ + I– + e– CuI)
2Cu2+(aq) + 4I–(aq) 2CuI(s) + I2(aq)
48.
Cu(s) + 2HNO3(aq) + 2H+(aq) Cu2+(aq) + 2NO2(g) + 2H2O(l)
Mg(s) + 2HNO3(aq) Mg(NO3)2(aq) + H2(g)
49.
Answer depends on student choice of reaction.
50.
A salt bridge typically consists of a U–shaped tube filled with an inert electrolyte (one involving i
ons that are not part of the oxidation–reduction reaction). A salt bridge is used to complete the ele
ctrical circuit in a cell. Any method that allows transfer of charge without allowing bulk mixing o
f the solutions may be used (another common method is to set up one half–cell in a porous cup, w
hich is then placed in the beaker containing the second half–cell).
51.
In a galvanic cell, electrons flow from the anode (where oxidation occurs) to the cathode (where r
eduction occurs).
52.
Reduction takes place at the cathode and oxidation takes place at the anode.
417
Chapter 18: Oxidation–Reduction Reactions/Electrochemistry
53.
A diagram of the cell is shown below:
Ni2+(aq) ion is reduced; Al(s) is oxidized.
The reaction at the anode is Al(s) Al3+(aq) + 3e–.
The reaction at the cathode is Ni2+(aq) + 2e– Ni(s).
54.
A diagram of the cell is shown below:
Pb2+(aq) ion is reduced; Zn(s) is oxidized.
The reaction at the anode is Zn(s) Zn2+(aq) + 2e–.
The reaction at the cathode is Pb2+(aq) + 2e– Pb(s)
55.
The overall reaction is
Pb(s) + PbO2(s) + 2H2SO4(aq) 2PbSO4(s) + 2H2O(l)
in which Pb0(s) is oxidized to Pb2+ and PbIVO2 is reduced to Pb2+. This reaction can be reversed by
electrolysis of the mixture of water and PbSO4(s) (passing electrical energy into the mixture from
the outside).
56.
Cd + 2OH– Cd(OH)2 + 2e–
(oxidation)
NiO2 + 2H2O + 2e– Ni(OH)2 + 2OH– (reduction)
57.
Corrosion represents returning metals to the natural state (ore) and involves oxidation of the meta
l. Corrosion of a metal is undesirable because, as the metal is converted to its oxide, the bulk of t
418

Chapter 18: Oxidation–Reduction Reactions/Electrochemistry
he metal loses its strength, flexibility, and other metallic properties. If the metal were part of som
e constructed item, the item would slowly disintegrate.
58.
Aluminum is a very reactive metal when freshly isolated in the pure state. However, on standing f
or even a relatively short period of time, aluminum metal forms a thin coating of Al2O3 on its surf
ace from reaction with atmospheric oxygen. This coating of Al2O3 is much less reactive than the
metal and serves to protect the surface of the metal from further attack.
59.
Most steels contain additives such as chromium or nickel. These additives are able to form protec
tive oxide coatings on the surface of the steel that tend to prevent further oxidation.
60.
Chromium protects stainless steel by forming a thin coating of chromium oxide on the surface of
the steel, which prevents oxidation of the iron in the steel.
61.
In an electrolysis cell, an electric current from outside the cell is used to force an otherwise non-s
pontaneous oxidation–reduction reaction to occur. In a galvanic cell, a spontaneous oxidation–red
uction reaction is used as a source of electrical current.
62.
The main recharging reaction for the lead storage battery is
2PbSO4(s) + 2H2O(l) Pb(s) + PbO2(s) + 2H2SO4(aq).
A major side reaction is the electrolysis of water
2H2O(l) 2H2(g) + O2(g).
This results in production of an explosive mixture of hydrogen and oxygen, which accounts for
many accidents in recharging of such batteries.
63.
Aluminum is so reactive towards oxygen that there proved to be no convenient chemical reducing
agent which could reduce aluminum ores to the metal. Widespread commercial preparation of alu
minum was only possible when an electrolytic process was developed
64.
The balanced equation is 2H2O(l) 2H2(g) + O2(g). Oxygen is oxidized (going from –2 oxidatio
n state in water to zero oxidation state in the free element). Hydrogen is reduced (going from +1 o
xidation state in water to zero oxidation state in the free element). Heat is produced by burning th
e hydrogen gas produced by the electrolysis: since energy must be applied to water to electrolyze
it, energy is released when hydrogen gas produced by the electrolysis and oxygen gas combine to
form water in the fireplace.
65.
electrons
66.
loss; oxidation state
67.
gain, oxidation number
68.
electronegative
69.
charge
70.
An oxidizing agent is an atom, molecule, or ion that causes the oxidation of another species. Duri
ng this process, the oxidizing agent is reduced.
71.
oxidation, reduced
419

Chapter 18: Oxidation–Reduction Reactions/Electrochemistry
72.
lose
73.
equal
74.
separate from
75.
galvanic
76.
oxidation
77.
cathode
78.
reducing; oxidizing
79.
voltage or potential
80.
electrolysis; In a galvanic cell, chemical energy is converted to electrical energy by means of an o
xidation-reduction reaction. In electrolysis, electrical energy is used to produce a chemical change.
81.
zinc
82.
oxidation
83.
aluminum oxide
84.
a.
4Fe(s) + 3O2(g) 2Fe2O3(s)
iron is oxidized; oxygen is reduced
b.
2Al(s) + 3Cl2(g) 2AlCl3(s)
aluminum is oxidized; chlorine is reduced
c.
6Mg(s) + P4(s) 2Mg3P2(s)
magnesium is oxidized; phosphorus is reduced
85.
a.
zinc is oxidized; hydrogen (as H+ in HCl) is reduced
b.
copper(I) is both oxidized to copper(II) and reduced to copper(0)
c.
iron (as Fe2+) is oxidized to Fe3+; chromium(VI) (in Cr2O72–) is reduced to Cr3+
86.
a.
aluminum is oxidized; hydrogen is reduced
b.
hydrogen is reduced; iodine is oxidized
c.
copper is oxidized; hydrogen is reduced
420

Chapter 18: Oxidation–Reduction Reactions/Electrochemistry
87.
a.
CH2=CH2(g) + Cl2(g) ClCH2–CH2Cl(l)
carbon is oxidized (–2 to –1); chlorine is reduced (0 to –1)
Cl2 is the oxidizing agent; CH2=CH2 is the reducing agent
b.
CH2=CH2(g) + Br2(g) BrCH2–CH2Br(l)
carbon is oxidized (–2 to –1); bromine is reduced (0 to –1)
Br2 is the oxidizing agent; CH2=CH2 is the reducing agent
c.
CH2=CH2(g) + HBr(g) CH3–CH2Br(l)
The process appears not to involve electron transfer: there are no changes in oxidation
number.
d.
CH2=CH2(g) + H2(g) CH3–CH3
carbon is reduced (–3 to –3); hydrogen is oxidized (0 to +1)
hydrogen is the reducing agent; CH2=CH2 is the oxidizing agent
88.
a.
C3H8(g) + 5O2(g) 3CO2(g) + 4H2O(g)
b.
CO(g) + 2H2(g) CH3OH(l)
c.
SnO2(s) + 2C(s) Sn(s) + 2CO(g)
d.
C2H5OH(l) + 3O2(g) 2CO2(g) + 3H2O(g)
89.
a.
2MnO4–(aq) + 6H+(aq) + 5H2O2(aq) 2Mn2+(aq) + 8H2O(l) + 5O2(g)
b.
6Cu+(aq) + 6H+(aq) + BrO3–(aq) 6Cu2+(aq) + Br–(aq) + 3H2O(l)
c.
2HNO2(aq) + 2H+(aq) + 2I–(aq) 2NO(g) + I2(aq) + 2H2O(l)
90.
Each of these reactions involves a metallic element in the form of the free element on one side of
the equation; on the other side of the equation, the metallic element is combined in an ionic comp
ound. If a metallic element goes from the free metal to the ionic form, the metal is oxidized (loses
electrons).
a.
sodium is oxidized; oxygen is reduced
b.
iron is oxidized; hydrogen is reduced
c.
oxygen (O2–) is oxidized; aluminum (Al3+) is reduced (this reaction is the reverse of the
type discussed above)
d.
magnesium is oxidized; nitrogen is reduced
91.
Each of these reactions involves a metallic element in the form of the free element on one side of
the equation; on the other side of the equation, the metallic element is combined in an ionic comp
ound. If a metallic element goes from the free metal to the ionic form, the metal is oxidized (loses
electrons).
a.
zinc is oxidized; nitrogen is reduced
b.
cobalt is oxidized; sulfur is reduced
421

Chapter 18: Oxidation–Reduction Reactions/Electrochemistry
c.
potassium is oxidized; oxygen is reduced
d.
silver is oxidized; oxygen is reduced
92.
The rules for assigning oxidation states are given in Section 18.2 of the text. The rule that applies
for each element in the following answers is given in parentheses after the element and its oxidati
on state.
a.
H +1 (Rule 4); N –3 (Rule 6)
b.
C +2 (Rule 6); O –2 (Rule 3)
c.
C +4 (Rule 6); O –2 (Rule 3)
d.
N +3 (Rule 6); F –1 (Rule 5)
93.
The rules for assigning oxidation states are given in Section 18.2 of the text. The rule that applies
for each element in the following answers is given in parentheses after the element and its oxidati
on state.
a.
P +3 (Rule 6); Br –1 (Rule 5)
b.
C –(8/3) (Rule 6); H +1 (Rule 4)
c.
K +1 (Rule 2); Mn +7 (Rule 6); O –2 (Rule 3)
d.
C 0 (Rule 6); H +1 (Rule 4); O –2 (Rule 3)
94.
The rules for assigning oxidation states are given in Section 18.2 of the text. The rule that applies
for each element is that given in parentheses after the element and its oxidation state.
a.
Mn +4 (Rule 6); O –2 (Rule 3)
b.
Ba +2 (Rule 2); Cr +6 (Rule 6); O –2 (Rule 3)
c.
H +1 (Rule 4); S +4 (Rule 6); O –2 (Rule 3)
d.
Ca +2 (Rule 2); P +5 (Rule 6); O –2 (Rule 3)
95.
The rules for assigning oxidation states are given in Section 18.2 of the text. The rule that applies
for each element is that given in parentheses after the element and its oxidation state.
a.
Cr +3 (Rule 6); Cl –1 (Rule 2)
b.
K +1 (Rule 2); Cr +6 (Rule 6); O –2 (Rule 3)
c.
K +1 (Rule 2); Cr +6 (Rule 6); O –2 (Rule 3)
d.
Cr +2 (Rule 6);C 0 (Rule 7); H +1 (Rule 4); O –2 (Rule 3)
For chromous acetate, first the oxidation state of carbon in the acetate ion, C2H3O2–, is
determined by Rule 7 (the sum of the oxidation numbers must equal the charge on the
ion), then the oxidation state of Cr may be determined by Rule 6).
96.
The rules for assigning oxidation states are given in Section 18.2 of the text. The rule that applies
for each element is that given in parentheses after the element and its oxidation state.
a.
Bi +3 (Rule 7); O –2 (Rule 3)
b.
P +5 (Rule 7); O –2 (Rule 3)
422

Chapter 18: Oxidation–Reduction Reactions/Electrochemistry
c.
N +3 (Rule 7); O –2 (Rule 3)
d.
Hg +1 (Rule 7)
97.
a.
C(s) + O2(g) CO2(g)
carbon is oxidized (0 to +4); oxygen is reduced (0 to –2)
b.
2CO(g) + O2(g) 2CO2(g)
carbon (of CO) is oxidized (+2 to +4); oxygen (of O2) is reduced (0 to –2)
c.
CH4(g) + 2O2(g) CO2(g) + 2H2O(g)
carbon (of CH4) is oxidized (–4 to +4); oxygen (of O2) is reduced (0 to –2)
d.
C2H2(g) + 2H2(g) C2H6(g)
hydrogen (of H2) is oxidized (0 to +1); carbon (of C2H2) is reduced (–1 to –3)
98.
a.
2B2O3(s) + 6Cl2(g) 4BCl3(l) + 3O2(g)
oxygen is oxidized (–2 to 0); chlorine is reduced (0 to –1)
b.
GeH4(g) + O2(g) Ge(s) + 2H2O(g)
germanium is oxidized (–4 to 0); oxygen is reduced (0 to –2)
c.
C2H4(g) + Cl2(g) C2H4Cl2(l)
carbon is oxidized –2 to –1); chlorine is reduced (0 to –1)
d.
O2(g) + 2F2(g) 2OF2(g)
oxygen is oxidized (0 to +2); fluorine is reduced (0 to –1)
99.
a.
I–(aq) I2(s)
Balance iodine: 2I–(aq) I2(s)
Balance charge: 2I–(aq) I2(s) + 2e–
Balanced half–reaction: 2I–(aq) I2(s) + 2e–
b.
O2(g) O2–(s)
Balance oxygen: O2(g) 2O2–(s)
Balance charge: O2(g) + 4e– 2O2–(s)
Balanced half–reaction: O2(g) + 4e– 2O2–(s)
c.
P4(s) P3–(s)
Balance phosphorus: P4(s) 4P3–(s)
Balance charge: P4(s) + 12e– 4P3–(s)
Balanced half–reaction: P4(s) + 12e– 4P3–(s)
423

Chapter 18: Oxidation–Reduction Reactions/Electrochemistry
d.
Cl2(g) Cl–(aq)
Balance chlorine: Cl2(g) 2Cl–(aq)
Balance charge: Cl2(g) + 2e– 2Cl–(aq)
Balanced half–reaction: Cl2(g) + 2e– 2Cl–(aq)
100.
a.
SiO2(s) Si(s)
Balance oxygen: SiO2(s) Si(s) + 2H2O(l)
Balance hydrogen: SiO2(s)+ 4H+(aq) Si(s) + 2H2O(l)
Balance charge: SiO2(s)+ 4H+(aq) + 4e– Si(s) + 2H2O(l)
Balanced half–reaction: SiO2(s)+ 4H+(aq) + 4e– Si(s) + 2H2O(l)
b.
S(s) H2S(g)
Balance hydrogen: S(s) + 2H+(aq) H2S(g)
Balance charge: S(s) + 2H+(aq) + 2e– H2S(g)
Balanced half–reaction: S(s) + 2H+(aq) + 2e– H2S(g)
c.
NO3–(aq) HNO2(aq)
Balance oxygen: NO3–(aq) HNO2(aq) + H2O(l)
Balance hydrogen: NO3–(aq) + 3H+(aq) HNO2(aq) + H2O(l)
Balance charge: NO3–(aq) + 3H+(aq) + 2e– HNO2(aq) + H2O(l)
Balanced half–reaction: NO3–(aq) + 3H+(aq) + 2e– HNO2(aq) + H2O(l)
d.
NO3–(aq) NO(g)
Balance oxygen: NO3–(aq) NO(g) + 2H2O(l)
Balance hydrogen: NO3–(aq) + 4H+(aq) NO(g) + 2H2O(l)
Balance charge: NO3–(aq) + 4H+(aq) + 3e– NO(g) + 2H2O(l)
Balanced half–reaction: NO3–(aq) + 4H+(aq) + 3e– NO(g) + 2H2O(l)
101.
For simplicity, the physical states of the substances have been omitted until the final balanced equ
ation is given.
a.
I–(aq) + MnO4–(aq) I2(aq) + Mn2+(aq)
I– I2
Balance iodine: 2I– I2
Balance charge: 2I– I2 + 2e–
MnO4– Mn2+
Balance oxygen: MnO4– Mn2+ + 4H2O
Balance hydrogen: 8H+ + MnO4– Mn2+ + 4H2O
Balance charge: 8H+ + MnO4– + 5e– Mn2+ + 4H2O
Combine the half–reactions:
424

Chapter 18: Oxidation–Reduction Reactions/Electrochemistry
5 × (2I– I2 + 2e–)
2 × (8H+ + MnO4– + 5e– Mn2+ + 4H2O)
16H+(aq) + 2MnO4–(aq) + 10I–(aq) 2Mn2+(aq) + 8H2O(l) + 5I2(aq)
b.
S2O82– + Cr3+ SO42– + Cr2O72–
S2O82– SO42–
Balance sulfur: S2O82– 2SO42–
Balance charge: S2O82– + 2e– 2SO42–
Cr3+ Cr2O72–
Balance chromium: 2Cr3+ Cr2O72–
Balance oxygen: 7H2O + 2Cr3+ Cr2O72–
Balance hydrogen: 7H2O + 2Cr3+ Cr2O72– + 14H+
Balance charge: 7H2O + 2Cr3+ Cr2O72– + 14H+ + 6e–
Combine the half–reactions:
3 × (S2O82– + 2e– 2SO42–)
7H2O + 2Cr3+ Cr2O72– + 14H+ + 6e–
7H2O(l) + 2Cr3+(aq) + 3S2O82–(aq) Cr2O72–(aq) + 14H+(aq) + 6SO42–(aq)
c.
BiO3– + Mn2+ Bi3+ + MnO4–
BiO3– Bi3+
Balance oxygen: BiO3– Bi3+ + 3H2O
Balance hydrogen: 6H+ + BiO3– Bi3+ + 3H2O
Balance charge: 6H+ + BiO3– + 2e– Bi3+ + 3H2O
Mn2+ MnO4–
Balance oxygen: 4H2O + Mn2+ MnO4–
Balance hydrogen: 4H2O + Mn2+ MnO4– + 8H+
Balance charge: 4H2O + Mn2+ MnO4– + 8H+ + 5e–
Combine the half–reactions:
5 × (6H+ + BiO3– + 2e– Bi3+ + 3H2O)
2 × (4H2O + Mn2+ MnO4– + 8H+ + 5e–)
2Mn2+(aq) + 14H+(aq) + 5BiO3–(aq) 2MnO4–(aq) + 5Bi3+(aq) + 7H2O(l)
102.
The correct answer is d. Al3+(aq) ion is reduced. Mg(s) is oxidized. Reduction occurs at the cath
ode and oxidation occurs at the anode. The reaction at the cathode is 2Al3+(aq) + 6e– 2Al(s). T
he reaction at the anode is 3Mg(s) 3Mg2+(aq) + 6e–.
425
Chapter 18: Oxidation–Reduction Reactions/Electrochemistry
103.
A diagram of the cell is shown below:
Cu2+(aq) ion is reduced; Mg(s) is oxidized.
The reaction at the anode is Mg(s) Mg2+(aq) + 2e–.
The reaction at the cathode is Cu2+(aq) + 2e– Cu(s).
104.
Notice that both dyes include “C16N2H10O2”. Since leucoindigo is Na2C16N2H10O2, the “C16N2H10O
2” portion has a 2– charge while indigo (C16N2H10O2) is neutral. Since the sum of the oxidation sta
tes equals the charge, the oxidation state of one or more of the elements must increase, thus the m
olecule must be oxidized.
105.
Oxidation State
S in MgSO4
+6
Pb in PbSO4
+2
O in O2
0
Ag in Ag
0
Cu in CuCl2
+2
426