CHAPTER 11
Modern Atomic Theory
1.
positively; negatively
2.
Rutherford was not able to determine where the electrons were in the atom or what they were doi
ng.
3.
Electromagnetic radiation is radiant energy that travels through space with wavelike behavior at t
he speed of light.
4.
The different forms of electromagnetic radiation are similar in that they all exhibit the same type
of wave-like behavior and are propagated through space at the same speed (the speed of light). Th
e types of electromagnetic radiation differ in their frequency (and wavelength) and in the resultin
g amount of energy carried per photon.
5.
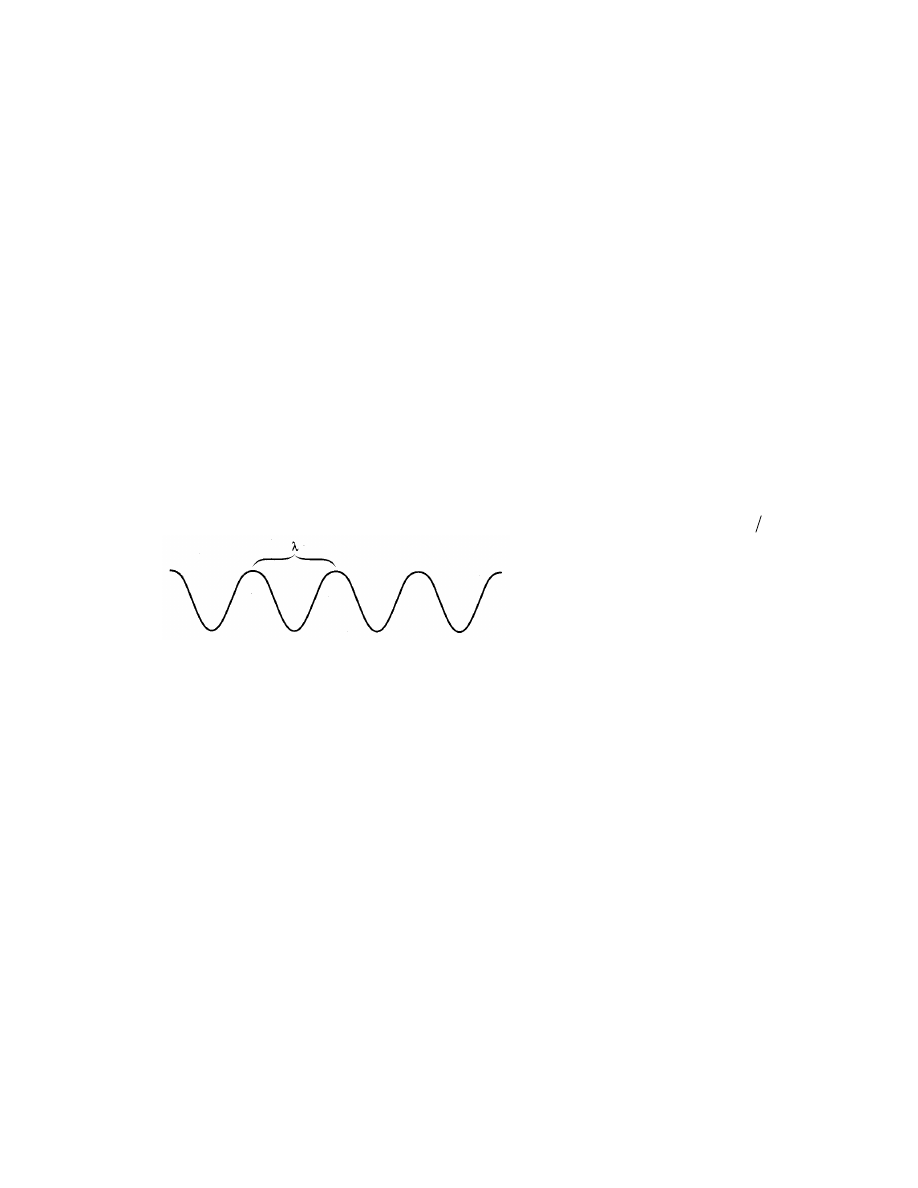
The wavelength represents the distance between corresponding points on two successive
waves. The energy of a photon is inversely proportional to the wavelength (E = hc )
6.
The speed of electromagnetic radiation represents how fast a given wave moves through space. T
he frequency of electromagnetic radiation represents how many complete cycles of the wave pass
a given point per second. These two concepts are not the same.
7.
Ultraviolet radiation is of shorter wavelength than visible light, and therefore is of higher energy t
han visible light.
8.
The greenhouse gases do not absorb light in the visible wavelengths, enabling this light to pass
through the atmosphere and continue to warm the earth, keeping the earth much warmer than it
would be without these gases. The earth, in turn, emits infrared radiation which is absorbed by the
greenhouse gases and which is re-emitted in all directions. As we increase our use of fossil fuels,
the level of CO2 in the atmosphere is increasing gradually, but significantly. An increase in the
level of CO2 will warm the earth further, eventually changing the weather patterns on the earth’s
surface and melting the polar ice caps.
9.
The atoms in salts emit light of characteristic wavelengths because of how their internal electron s
tructures interact with the applied energy. Each element’s atoms have a different electron structur
e, so each atom emits light differently from all other atoms.
10.
exactly equal to
11.
The ground state represents the lowest-energy state of the atom.
225

Chapter 11: Modern Atomic Theory
12.
A photon having an energy corresponding to the energy difference between the two states is emitt
ed by an atom in an excited state when it returns to its ground state.
13.
The energy of a photon is given by /
E
hc
and therefore short wavelength light carries more
energy per photon.
14.
absorbs
15.
The emission of light by excited atoms has been the key interconnection between the macroscopic
world we can observe and measure, and with what is happening on a microscopic basis within an
atom. Excited atoms emit light (which we can measure) because of changes in the microscopic st
ructure of the atom. By studying the emissions of atoms we can trace back to what happened insi
de the atom.
16.
When excited hydrogen atoms emit their excess energy, the photons of radiation emitted are alwa
ys of exactly the same wavelength and energy. We consider this to mean that the hydrogen atom
possesses only certain allowed energy states, and that the photons emitted correspond to the atom
changing from one of these allowed energy states to another of the allowed energy state. The ener
gy of the photon emitted corresponds to the energy difference in the allowed states. If the hydroge
n atom did not possess discrete energy levels, then we would expect the photons emitted to have r
andom wavelengths and energies.
17.
transitions of electrons
18.
The energy of an emitted photon is identical to the energy change within the atom that gave rise t
o the emitted photon.
19.
quantized
20.
Energy is emitted only at wavelengths corresponding to the specific transitions for the electron
among the energy levels of hydrogen.
21.
Bohr envisioned electrons as moving in circular orbits corresponding to the various allowed energ
y levels. He suggested that the electron could jump to a different orbit by absorbing or emitting a
photon of light with exactly the correct energy content (corresponding to the difference in energy
between the orbits).
22.
The electron moves to an orbit farther from the nucleus of the atom.
23.
Bohr suggested that the electron could jump to a different orbit by absorbing or emitting a photon
of light with exactly the correct energy content (corresponding to the difference in energy betwee
n the orbits).
As the energy levels of a given atom were fixed and definite, then the atom should always emit
energy at the same discrete wavelengths.
24.
Bohr’s theory explained the experimentally observed line spectrum of hydrogen exactly. Bohr’s t
heory was ultimately discarded because when attempts were made to extend the theory to atoms o
ther than hydrogen, the calculated properties did not correspond closely to experimental measure
ments.
226

Chapter 11: Modern Atomic Theory
25.
Schrödinger and de Broglie reasoned that, because light seems to have both wave and particle cha
racteristics (it behaves simultaneously as a wave and as if it were a stream of particles), that perha
ps the electron might exhibit both of these characteristics. That is, although the electron behaves
as a discrete particle, perhaps the properties of the electron in the atom could be treated as if they
were wavelike.
26.
An orbit represents a definite, exact circular pathway around the nucleus in which an electron can
be found. An orbital represents a region of space in which there is a high probability of finding th
e electron.
27.
Schrödinger’s mathematical treatment could only describe the movement of the electron through t
he atom in terms of the probability of finding the electron in given regions of space within the ato
m, but not at a particular point within the atom at a particular time. Any attempt to determine the
exact position of the electron within an atom experimentally would, in fact, disturb the electron fr
om wherever it had been.
28.
The firefly analogy is intended to demonstrate the concept of a probability map for electron densit
y. In the wave mechanical model of the atom, we cannot say specifically where the electron is in
the atom, we can only say where there is a high probability of finding the electron. The analogy is
to imagine a time-exposure photograph of a firefly in a closed room. Most of the time, the firefly
will be found near the center of the room.
29.
Although the probability of finding the electron decreases at greater distances from the nucleus, t
he probability of finding it even at great distances from the nucleus never becomes exactly zero.
The probability becomes less and less as you move away from the nucleus, similar to the way the
atmosphere becomes thinner and thinner as you move away from the surface of the earth.
30.
(b)
31.
The 2s orbital is similar in shape to the 1s orbital, but is larger.
32.
The p orbitals, in general, have two lobes and are sometimes described as having a “dumbbell” sh
ape. The 2p and 3p orbitals are similar in shape, and in fact there are three equivalent 2p or 3p orb
itals in the 2p or 3p subshell. The orbitals differ in size, mean distance from the nucleus, and ener
gy.
33.
farther from
34.
excited
35.
The other orbitals serve as the excited states of the hydrogen atom. When energy of the right freq
uency is applied to the hydrogen atom, the electron can move from its normal orbital (ground stat
e) to one of the other orbitals (excited states). Later on, the electron can move back to its normal o
rbital and release the absorbed energy as light.
227

Chapter 11: Modern Atomic Theory
36.
Value of n
Possible subshells
1
1s
2
2s, 2p
3
3s, 3p, 3d
4
4s, 4p, 4d, 4f
37.
electron spin
38.
Electrons have an intrinsic spin (they spin on their own axes). Geometrically, there are only two
senses possible for spin (clockwise or counter-clockwise). This means only two electrons can occ
upy an orbital, with the opposite sense or direction of spin. This idea is called the Pauli Exclusio
n Principle.
39.
The higher the value of the principal quantum number, n, the higher the energy of the principal en
ergy level.
40.
increases; as you move out from the nucleus, there is more space and room for more sublevels.
41.
two
42.
opposite
43.
Choices a, b, and d are possible; choice c is not possible because the d subshells do not begin unti
l the n = 3 orbit.
44.
(a) and (c); f orbitals begin at the fourth energy level and a d orbitals begin at the third energy lev
el
45.
The 1s orbital is closest to the nucleus and lowest in energy, so it is always filled first.
46.
When a hydrogen atom is in its ground state, the electron is found in the 1s orbital. The 1s orbital
has the lowest energy of all the possible hydrogen orbitals.
47.
Valence electrons are those in the outermost (highest) principal energy level of an atom. These el
ectrons are especially important because they are at the “outside edge” of an atom, and are those e
lectrons that are “seen” by other atoms and can interact with the electrons of another atom in a ch
emical reaction.
48.
The elements in a given vertical column of the periodic table have the same valence electron conf
iguration. Having the same valence electron configuration causes the elements in a given group to
have similar chemical properties.
49.
a.
1s2 2s2 2p6 3s2
b.
1s2 2s1
c.
1s2 2s2 2p4
d.
1s2 2s2 2p6 3s2 3p4
228

Chapter 11: Modern Atomic Theory
50.
Just count the electrons to get the atomic number of the element.
a.
silicon
b.
beryllium
c.
neon
d.
argon
51.
a.
1s2 2s2 2p6 3s2 3p3
b.
1s2 2s2 2p6 3s2 3p6 4s2
c.
1s2 2s2 2p6 3s2 3p6 4s1
d.
1s2 2s22p1
52.
Just count the electrons to get the atomic number of the element.
a.
selenium
b.
scandium
c.
sulfur
d.
iodine
53.
a.
1s( )
b.
1s( ) 2s( ) 2p( )( )( )
c.
1s( ) 2s( ) 2p( )( )( ) 3s( ) 3p( )( )( ) 4s( ) 3d( )(
)( )( )( ) 4p( )( )( )
d.
1s( ) 2s( ) 2p( )( )( ) 3s( ) 3p( )( )( ) 4s( ) 3d( )(
)( )( ( ) 4p( )( )( ) 5s( ) 4d( )( )( )( )( ) 5p(
)( )( )
54.
a.
1s() 2s() 2p()()() 3s() 3p()( )( )
b.
1s() 2s() 2p()()() 3s() 3p()()()
c.
1s() 2s() 2p()()() 3s() 3p()()() 4s()
3d()()()()() 4p()()()
d.
1s() 2s() 2p()()() 3s() 3p()()()
55.
Group 2 (ns2) and Group 8 (ns2 np6) are the only groups where all electrons are paired.
56.
Specific answers depend on student choice of elements. Any Group 1 element would have one
valence electron. Any Group 3 element would have three valence electrons. Any Group 5 element
would have five valence electrons. Any Group 7 element would have seven valence electrons.
57.
This belief is based on the experimental properties of K and Ca. The physical and chemical prope
rties of K are like those of the other Group 1 elements; Ca’s properties are similar to the other Gr
oup 2 elements.
229

Chapter 11: Modern Atomic Theory
58.
The properties of Rb and Sr suggest that they are members of Groups 1 and 2, respectively, and s
o must be filling the 5s orbital. The 5s orbital is lower in energy (and fills before) the 4d orbitals.
59.
a.
[Ar] 4s2 3d10 4p3
b.
[Ar] 4s2 3d2
c.
[Kr] 5s2
d.
[Ne] 3s2 3p5
60.
a.
aluminum
b.
potassium
c.
bromine
d.
tin
61.
a.
[Ar] 4s2 3d1
b.
[Kr] 5s2 4d1
c.
[Xe] 6s2 5d1
d.
[Rn] 7s2 6d1
62.
a.
[Kr] 5s1: 1 valence electron
b.
[Ar] 4s2 3d10 4p3: 5 valence electrons (d electrons are not counted as valence electrons)
c.
[Ne] 3s2 3p1: 3 valence electrons
d.
[Ar] 4s2 3d8: 2 valence electrons (d electrons are not counted as valence electrons)
63.
a.
eight
b.
three
c.
five
d.
six
64.
a.
[Kr] 5s2 4d6: 6 4d electrons
b.
[Kr] 5s2 4d8: 8 4d electrons
c.
[Kr] 5s2 4d10 5p2: 10 4d electrons
d.
[Ar] 4s2 3d6: 0 4d electrons
65.
The position of the element (both in terms of vertical column and horizontal row) indicates which
set of orbitals is being filled last.
a.
7s
b.
5p
c.
5d
d.
6p
230

Chapter 11: Modern Atomic Theory
66.
a.
[Rn]7s26d15f3
b.
[Ar]4s23d5
c.
[Xe]6s24f145d10
d.
[Rn]7s1
67.
The valence electrons are those beyond the noble gas “core.”
a.
[Kr] 5s1
b.
[Xe] 6s2
c.
[Ar] 4s2 3d2
d.
[Ar] 4s2 3d10 4p2
68.
[Rn] 7s2 5f14 6d5
69.
Some typical properties of metals are: a lustrous appearance, the ability to be pounded into sheets
(malleability) or pulled into wires (ductility), and the ability to conduct heat and electricity. Non
metals typically have a non-shiny appearance, are brittle, and do not conduct heat or electricity w
ell. There are exceptions to these general properties: for example, graphite (a form of the nonmeta
l carbon) conducts electricity well.
70.
The metallic elements lose electrons and form positive ions (cations); the nonmetallic elements ga
in electrons and form negative ions (anions). Remember that the electron itself is negatively charg
ed.
71.
The Group 1 metals are all highly reactive, and all form 1+ ions almost exclusively when they rea
ct. Physically, these metals are soft (they can be cut with a knife) and very low in density. Becau
se of their high reactivity, these metals tend to be found with a coating of the metal oxide that hid
es their metallic luster (this luster can be seen, however, if a fresh surface of the metal is exposed).
72.
All exist as diatomic molecules (F2, Cl2, Br2, I2); all are nonmetals; all have relatively high electro
negativities; all form 1- ions in reacting with metallic elements.
73.
Cs: The valence electron is farther from the nucleus than in Li or K.
74.
Elements at the left of a period (horizontal row) lose electrons more readily; at the left of a period
(given principal energy level) the nuclear charge is the smallest and the electrons are least tightly
held.
75.
The nonmetallic elements are clustered at the upper right side of the periodic table. These element
s are effective at pulling electrons from metallic elements for several reasons. First, these element
s have little tendency to lose electrons themselves (they have high ionization energies). Secondly,
the atoms of these elements tend to be small in size, which means that electrons can be pulled in s
trongly since they can get closer to the nucleus. Finally, if these atoms gain electrons, they can ap
proach the electronic configuration of the following noble gas elements (see Chapter 12 for why t
he electronic configuration of the noble gases are desirable for other atoms to attain).
231

Chapter 11: Modern Atomic Theory
76.
The elements of a given period (horizontal row) have valence electrons in the same principal ener
gy level. Nuclear charge, however, increases across a period going from left to right. Atoms at the
left side have smaller nuclear charges and hold onto their valence electrons less tightly.
77.
Metalloids are elements that have both metallic and non-metallic properties. They are located alo
ng either side of the “stair-step” towards the right side of the periodic table.
78.
When substances absorb energy the electrons become excited (move to higher energy levels). Up
on returning to the ground state, energy is released, some of which is in the visible spectrum. Sin
ce we see colors, this tells us that only certain wavelengths of light are released, which means that
only certain transitions are allowed. This is what is meant by quantized energy levels. If all wav
elengths of light were emitted we would see white light.
79.
For most elements, the chemical activity is reflected in the ease with which the element gains or l
oses electrons
a.
Li (the less reactive metals are further up in a group)
b.
At (the less reactive nonmetals are at the bottom of a group)
c.
Be (the less reactive metals are further up in a group)
d.
Po (the less reactive elements are at the bottom of a group)
80.
Ionization energies decrease in going from top to bottom within a vertical group; ionization energ
ies increase in going from left to right within a horizontal period.
a.
Li
b.
Ca
c.
Cl
d.
S
81.
Atomic size increases in going from top to bottom within a vertical group; atomic size decreases i
n going from left to right within a horizontal period.
a.
Xe < Sn < Sr < Rb
b.
He < Kr < Xe < Rn
c.
At < Pb < Ba < Cs
82.
Atomic size increases in going from top to bottom within a vertical group; atomic size decreases i
n going from left to right within a horizontal period.
a.
Na
b.
S
c.
N
d.
F
83.
The highest-energy photons are responsible for the line at 410 nm; the lowest-energy photons giv
e rise to the line at 656 nm.
84.
speed of light
232

Chapter 11: Modern Atomic Theory
85.
visible
86.
photons
87.
ground
88.
quantized
89.
orbits
90.
orbital
91.
valence
92.
transition metal
93.
frequency
94.
spins
95.
a.
[Ne] 3s( ) 3p(
)(
)(
)
P is expected to be paramagnetic; three unpaired 3p electrons
b.
[Kr] 5s( ) 4d( )( )( )( )( ) 5p( )( )(
)
I is expected to be paramagnetic; one unpaired 5p electron
c.
[Ar] 4s( ) 3d( )( )( )( )( ) 4p(
)(
)( )
Ge is expected to be paramagnetic; two unpaired 4p electrons
96.
a.
1s2 2s2 2p6 3s2 3p6 4s1
[Ar] 4s1
1s( ) 2s( ) 2p( )( )( ) 3s( ) 3p( )( )( ) 4s(
)
b.
1s2 2s2 2p6 3s2 3p6 4s2 3d2
[Ar] 4s2 3d2
1s( ) 2s( ) 2p( )( )( ) 3s( ) 3p( )( )( ) 4s( )
3d(
)(
)( )( )( )
c.
1s2 2s2 2p6 3s2 3p2
[Ne] 3s2 3p2
1s( ) 2s( ) 2p( )( )( ) 3s( ) 3p(
)(
)( )
d.
1s2 2s2 2p6 3s2 3p6 4s2 3d6
[Ar] 4s2 3d6
1s( ) 2s( ) 2p( )( )( ) 3s( ) 3p( )( )( ) 4s( ) 3d( )(
)(
)(
)(
)
e.
1s2 2s2 2p6 3s2 3p6 4s2 3d10
[Ar] 4s2 3d10
1s( ) 2s( ) 2p( )( )( ) 3s( ) 3p( )( )( ) 4s( ) 3d( )( )
( )( )( )
233

Chapter 11: Modern Atomic Theory
97.
a.
1s2 2s2 2p6 3s2 3p6 4s2 3d1
1s( ) 2s( ) 2p( )( )( ) 3s( ) 3p( )( )( ) 4s( )
3d(
)( )( )()( )
[Ar] 4s2 3d1
b.
1s2 2s2 2p6 3s2 3p3
1s( ) 2s( ) 2p( )( )( ) 3s( ) 3p(
)(
)(
)
[Ne] 3s2 3p3
c.
1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6
1s( ) 2s( ) 2p( )( )( ) 3s( ) 3p( )( )( ) 4s( ) 3d( )( )
( )( )( ) 4p( )( )( )
[Kr] is itself a noble gas
d.
1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2
1s( ) 2s( ) 2p( )( )( ) 3s( ) 3p( )( )( ) 4s( ) 3d( )( )
( )( )( ) 4p( )( )( ) 5s( )
[Kr] 5s2
e.
1s2 2s2 2p6 3s2 3p6 4s2 3d10
1s( ) 2s( ) 2p( )( )( ) 3s( ) 3p( )( )( ) 4s( ) 3d( )( )
( )( )( )
[Ar] 4s2 3d10
98.
a.
ns2
b.
ns2 np5
c.
ns2 np4
d.
ns1
e.
ns2 np4
99.
a.
four (two if the d electrons are not counted.)
b.
seven
c.
two
d.
seven (two if the d electrons are not counted.)
100.
a.
mv
h
34
31
8
1
6.63 10
J s
(9.1 10 kg)[0.90 (3.00 10 m s )
= 2.7 × 10
–12 m (0.0027 nm)
234

Chapter 11: Modern Atomic Theory
b.
4.4 × 10–34 m
c.
2 × 10–35 m
The wavelengths for the ball and the person are infinitesimally small, whereas the
wavelength for the electron is nearly the same order of magnitude as the diameter of a
typical atom.
101.
3.00 × 108 m/sec
102.
Light is emitted from the hydrogen atom only at certain fixed wavelengths. If the energy levels of
hydrogen were continuous, a hydrogen atom would emit energy at all possible wavelengths.
103.
As an electron moves to a higher-number principal energy level, the electron’s mean distance fro
m the nucleus increases, thereby decreasing the attractive force between the electron and the nucl
eus.
104.
[Ar]4s23d7: …3d()()( )( )( ); 3 unpaired electrons
105.
Orbitals in the 1s and 2s subshells can only contain two electrons.
106.
a.
[Ne] 3s2 3p4; 16 electrons in this ground state atom which corresponds to sulfur.
b.
[He] 2s1 2p4; 7 electrons in this excited state atom which corresponds to nitrogen. One
electron was excited from the 2s to 2p.
c.
[Ar] 4s2 3d10 4p5; 35 electrons in this ground state ion. A charge of –1 means an electron
was gained, thus making the ion Se– which corresponds to 34 protons.
107.
Electrons are negatively charged particles that repel each other. By placing the three electrons in
separate 2p orbitals (oriented at 90 to each other in space), the repulsion among the electrons is
minimized. The electrons can also have the same spin if they are in separate orbitals but this is no
t discussed at length in the text.
108.
a.
1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5
b.
1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6
c.
1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2
d.
1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p4
109.
a.
1s( ) 2s( ) 2p( )( )( ) 3s( ) 3p( )( )( ) 4s( )
3d( )( )( )( )( )
b.
1s( ) 2s( ) 2p( )( )( ) 3s( ) 3p( )( )( )
c.
1s( ) 2s( ) 2p( )( )( ) 3s( ) 3p( )( )( ) 4s( )
d.
1s( ) 2s( ) 2p( )( )( )
235

Chapter 11: Modern Atomic Theory
110.
a.
five (2s, 2p)
b.
seven (3s, 3p)
c.
one (3s)
d.
three (3s, 3p)
111.
transition metals
112.
a.
ns2 np3
b.
ns1
c.
ns2 np5
d.
ns2 np4
e.
ns2
113.
a.
[Ar] 4s2 3d2
b.
[Ar] 4s2 3d10 4p4
c.
[Kr] 5s2 4d10 5p3
d.
[Kr] 5s2
114.
(a) < (c) < (b); (a) corresponds to the element Xe. (c) corresponds to the element Sb. (b) correspo
nds to the element In. Atomic size increases from right to left across a row on the periodic table.
Xe < Sb < In
115.
a.
[Ar] 4s2 3d8
b.
[Kr] 5s2 4d3 (actually [Kr] 5s1 4d4 for reasons beyond text)
c.
[Xe] 6s2 4f14 5d2
d.
[Xe] 6s2 4f14 5d10 6p5
116.
metals, low; nonmetals, high
117.
a.
B and Al are both very reactive
b.
Na
c.
F
118.
Atomic size increases in going from top to bottom within a vertical group; atomic size decreases i
n going from left to right within a horizontal period.
a.
Ca
b.
P
c.
K
236

Chapter 11: Modern Atomic Theory
119.
2f: 0 electrons
2dxy: 0 electrons
3p: 6 electrons
5dyz: 2 electrons
4p: 6 electrons
120.
(b), (c), and (e)
121.
Ca: 1s2 2s2 2p6 3s2 3p6 4s2
B: 1s2 2s2 2p1
H: 1s1
S: 1s2 2s2 2p6 3s2 3p4
Be: 1s2 2s2
122.
a.
Te
b.
Ge
c.
F (9 total electrons)
123.
K: [Ar] 4s1
Be: [He] 2s2
Zr: [Kr] 5s2 4d2
Se: [Ar] 4s2 3d10 4p4
C: [He] 2s2 2p2
124.
F and B: B is the larger atom.
C and N: C is the larger atom.
B and Al: Al is the larger atom.
125.
He and Kr: He has the larger ionization energy.
Na and Al: Al has the larger ionization energy.
Cl and I: Cl has the larger ionization energy.
126.
Electron configuration
Symbol
IE
AR
1s22s22p63s2
Mg
0.738
160
1s22s22p63s23p4
S
0.999
104
1s22s22p63s23p64s2
Ca
0.590
197
237